Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus
- PMID: 18945909
- PMCID: PMC2588436
- DOI: 10.1523/JNEUROSCI.2478-08.2008
Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus
Abstract
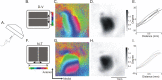
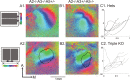
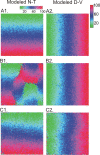
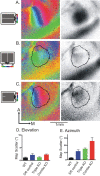
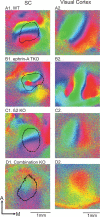
The orderly projections from retina to superior colliculus (SC) preserve a continuous retinotopic representation of the visual world. The development of retinocollicular maps depend on a combination of molecular guidance cues and patterned neural activity. Here, we characterize the functional retinocollicular maps in mice lacking the guidance molecules ephrin-A2, -A3, and -A5 and in mice deficient in both ephrin-As and structured spontaneous retinal activity, using a method of Fourier imaging of intrinsic signals. We find that the SC of ephrin-A2/A3/A5 triple knock-out mice contains functional maps that are disrupted selectively along the nasotemporal (azimuth) axis of the visual space. These maps are discontinuous, with patches of SC responding to topographically incorrect locations. The patches disappear in mice that are deficient in both ephrin-As and structured activity, resulting in a near-absence of azimuth map in the SC. These results indicate that ephrin-As guide the formation of functional topography in the SC, and patterned retinal activity clusters cells based on their correlated firing patterns. Comparison of the SC and visual cortical mapping defects in these mice suggests that although ephrin-As are required for mapping in both SC and visual cortex, ephrin-A-independent mapping mechanisms are more important in visual cortex than in the SC.
Figures


References
-
- Ballesteros JM, Sun C, Goloshchapov AV, Cheng H, Chalupa LM. Developing nAChR β2−/− mice manifest retinal waves of action potentials as well as altered retinogeniculate projection patterns. Soc Neurosci Abstr. 2007;33:346–21.
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. - PMC - PubMed
-
- Brown A, Yates PA, Burrola P, Ortuño D, Vaidya A, Jessell TM, Pfaff SL, O'Leary DD, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102:77–88. - PubMed
-
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
