Nigral inhibition of GABAergic neurons in mouse superior colliculus
- PMID: 18945914
- PMCID: PMC6671385
- DOI: 10.1523/JNEUROSCI.3263-08.2008
Nigral inhibition of GABAergic neurons in mouse superior colliculus
Abstract
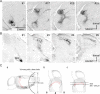
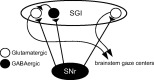
The current dominant concept for the control of saccadic eye movements by the basal ganglia is that release from tonic GABAergic inhibition by the substantia nigra pars reticulata (SNr) triggers burst firings of intermediate gray layer (SGI) neurons in the superior colliculus (SC) to allow saccade initiation. This hypothesis is based on the assumption that SNr cells inhibit excitatory projection neurons in the SGI. Here we show that nigrotectal fibers are connected to local GABAergic neurons in the SGI with a similar frequency to non-GABAergic neurons. This was accomplished by applying neuroanatomical tracing and slice electrophysiological experiments in GAD67-green fluorescent protein (GFP) knock-in mice, in which GABAergic neurons specifically express GFP. We also found that GABA(A), but not GABA(B), receptors subserve nigrotectal transmission. The present results revealed a novel aspect on the role of the basal ganglia in the control of saccades, e.g., the SNr not only regulates burst initiation but also modulates the spatiotemporal properties of premotor neurons via connections to local GABAergic neurons in the SC.
Figures



Similar articles
-
A circuit model for saccadic suppression in the superior colliculus.J Neurosci. 2011 Feb 9;31(6):1949-54. doi: 10.1523/JNEUROSCI.2305-10.2011. J Neurosci. 2011. PMID: 21307233 Free PMC article.
-
Nicotinic acetylcholine receptor subtypes involved in facilitation of GABAergic inhibition in mouse superficial superior colliculus.J Neurophysiol. 2005 Dec;94(6):3893-902. doi: 10.1152/jn.00211.2005. Epub 2005 Aug 17. J Neurophysiol. 2005. PMID: 16107532
-
Nigral GABAergic inhibition upon mesencephalic dopaminergic cell groups in rats.Eur J Neurosci. 2004 May;19(9):2399-409. doi: 10.1111/j.0953-816X.2004.03337.x. Eur J Neurosci. 2004. PMID: 15128394
-
GABAergic control of substantia nigra dopaminergic neurons.Prog Brain Res. 2007;160:189-208. doi: 10.1016/S0079-6123(06)60011-3. Prog Brain Res. 2007. PMID: 17499115 Review.
-
[Eye movement is controlled by basal ganglia-induced GABAergic inhibition].Rinsho Shinkeigaku. 1989 Dec;29(12):1515-8. Rinsho Shinkeigaku. 1989. PMID: 2517048 Review. Japanese.
Cited by
-
A distinct circuit for biasing visual perceptual decisions and modulating superior colliculus activity through the mouse posterior striatum.bioRxiv [Preprint]. 2024 Jul 31:2024.07.31.605853. doi: 10.1101/2024.07.31.605853. bioRxiv. 2024. PMID: 39372791 Free PMC article. Preprint.
-
The Substantia Nigra Pars Reticulata Modulates Error-Based Saccadic Learning in Monkeys.eNeuro. 2021 Apr 2;8(2):ENEURO.0519-20.2021. doi: 10.1523/ENEURO.0519-20.2021. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33707204 Free PMC article.
-
Neural mechanisms for the localization of unexpected external motion.Nat Commun. 2023 Sep 30;14(1):6112. doi: 10.1038/s41467-023-41755-z. Nat Commun. 2023. PMID: 37777516 Free PMC article.
-
Deep-Brain Stimulation for Basal Ganglia Disorders.Basal Ganglia. 2011 Jul 1;1(2):65-77. doi: 10.1016/j.baga.2011.05.001. Basal Ganglia. 2011. PMID: 21804953 Free PMC article.
-
α6* nicotinic acetylcholine receptor expression and function in a visual salience circuit.J Neurosci. 2012 Jul 25;32(30):10226-37. doi: 10.1523/JNEUROSCI.0007-12.2012. J Neurosci. 2012. PMID: 22836257 Free PMC article.
References
-
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol. 1990;302:143–158. - PubMed
-
- Basso MA, Liu P. Context-dependent effects of substantia nigra stimulation on eye movements. J Neurophysiol. 2007;97:4129–4142. - PubMed
-
- Basso MA, Pokorny JJ, Liu P. Activity of substantia nigra pars reticulata neurons during smooth pursuit eye movements in monkeys. Eur J Neurosci. 2005;22:448–464. - PubMed
-
- Bayer HM, Handel A, Glimcher PW. Eye position and memory saccade related responses in substantia nigra pars reticulata. Exp Brain Res. 2004;154:428–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
