Impact of sex and chronic resistance training on human patellar tendon dry mass, collagen content, and collagen cross-linking
- PMID: 18945950
- PMCID: PMC2636977
- DOI: 10.1152/ajpregu.90607.2008
Impact of sex and chronic resistance training on human patellar tendon dry mass, collagen content, and collagen cross-linking
Abstract
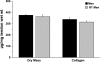
Collagen content and cross-linking are believed to be major determinants of tendon structural integrity and function. Sex and chronic resistance training have been shown to alter tendon function and may also alter the key structural features of tendon. Patellar tendon biopsies were taken from untrained men [n = 8, 1 repetition maximum (RM) = 53 +/- 3 kg], untrained women (n = 8, 1 RM = 29 +/- 2 kg), and resistance-trained (10 +/- 1 yr of training) men (n = 8, 1 RM = 71 +/- 6 kg). Biopsies were analyzed for dry mass, collagen content, and collagen cross-linking (hydroxylysylpyridinoline). We hypothesized that these elements of tendon structure would be lower in women than men, whereas chronic resistance training would increase these parameters in men. Tendon dry mass was significantly lower in women than men (343 +/- 5 vs. 376 +/- 8 microg dry mass/mg tendon wet wt, P < 0.01) and was not influenced by chronic resistance training (P > 0.05). The lower tendon dry mass in women tended to reduce (P = 0.08) collagen content per tendon wet weight. Collagen content of the tendon dry mass was not influenced by sex or resistance training (P > 0.05). Similarly, cross-linking of collagen was unaltered (P > 0.05) by sex or training. Although sex alters the water content of patellar tendon tissue, any changes in tendon function with sex or chronic resistance training in men do not appear to be explained by alterations in collagen content or cross-linking of collagen within the dry mass component of the tendon.
Figures

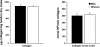
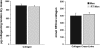
References
-
- Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl 703: 37–44, 1997. - PubMed
-
- Bergman BP, Miller SA. Equal opportunities, equal risks? Overuse injuries in female military recruits. J Public Health Med 23: 35–39, 2001. - PubMed
-
- Czarny-Ratajczak M, Latos-Bielenska A. Collagens, the basic proteins of the human body. J Appl Genet 41: 317–330, 2000. - PubMed
-
- Elliott DH Structure and function of mammalian tendon. Biol Rev Camb Philos Soc 40: 392–421, 1965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

