Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI
- PMID: 18945968
- PMCID: PMC2630279
- DOI: 10.1182/blood-2008-06-163675
Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI
Abstract
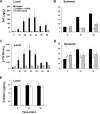
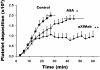
The protease thrombin is required for normal hemostasis and pathologic thrombogenesis. Since the mechanism of coagulation factor XI (FXI)-dependent thrombus growth remains unclear, we investigated the contribution of FXI to thrombus formation in a primate thrombosis model. Pretreatment of baboons with a novel anti-human FXI monoclonal antibody (aXIMab; 2 mg/kg) inhibited plasma FXI by at least 99% for 10 days, and suppressed thrombin-antithrombin (TAT) complex and beta-thromboglobulin (betaTG) formation measured immediately downstream from thrombi forming within collagen-coated vascular grafts. FXI inhibition with aXIMab limited platelet and fibrin deposition in 4-mm diameter grafts without an apparent increase in D-dimer release from thrombi, and prevented the occlusion of 2-mm diameter grafts without affecting template bleeding times. In comparison, pretreatment with aspirin (32 mg/kg) prolonged bleeding times but failed to prevent graft occlusion, supporting the concept that FXI blockade may offer therapeutic advantages over other antithrombotic agents in terms of bleeding complications. In whole blood, aXIMab prevented fibrin formation in a collagen-coated flow chamber, independent of factor XII and factor VII. These data suggest that endogenous FXI contributes to arterial thrombus propagation through a striking amplification of thrombin generation at the thrombus luminal surface.
Figures




References
-
- Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. - PubMed
-
- Moons AH, Peters RJ, Bijsterveld NR, et al. Recombinant nematode anticoagulant protein c2, an inhibitor of the tissue factor/factor VIIa complex, in patients undergoing elective coronary angioplasty. J Am Coll Cardiol. 2003;41:2147–2153. - PubMed
-
- Roque M, Reis ED, Fuster V, et al. Inhibition of tissue factor reduces thrombus formation and intimal hyperplasia after porcine coronary angioplasty. J Am Coll Cardiol. 2000;36:2303–2310. - PubMed
-
- Gershwin ME, Gude JK. Deep vein thrombosis and pulmonary embolism in congenital factor VII deficiency. N Engl J Med. 1973;288:141–142. - PubMed
-
- Asakai R, Chung DW, Davie EW, Seligsohn U. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991;325:153–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

