Adaptive antioxidant methionine accumulation in respiratory chain complexes explains the use of a deviant genetic code in mitochondria
- PMID: 18946048
- PMCID: PMC2575448
- DOI: 10.1073/pnas.0802779105
Adaptive antioxidant methionine accumulation in respiratory chain complexes explains the use of a deviant genetic code in mitochondria
Abstract
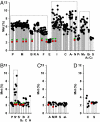
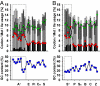
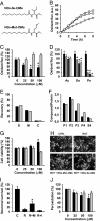
Humans and most other animals use 2 different genetic codes to translate their hereditary information: the standard code for nuclear-encoded proteins and a modern variant of this code in mitochondria. Despite the pivotal role of the genetic code for cell biology, the functional significance of the deviant mitochondrial code has remained enigmatic since its first description in 1979. Here, we show that profound and functionally beneficial alterations on the encoded protein level were causative for the AUA codon reassignment from isoleucine to methionine observed in most mitochondrial lineages. We demonstrate that this codon reassignment leads to a massive accumulation of the easily oxidized amino acid methionine in the highly oxidative inner mitochondrial membrane. This apparently paradoxical outcome can yet be smoothly settled if the antioxidant surface chemistry of methionine is taken into account, and we present direct experimental evidence that intramembrane accumulation of methionine exhibits antioxidant and cytoprotective properties in living cells. Our results unveil that methionine is an evolutionarily selected antioxidant building block of respiratory chain complexes. Collective protein alterations can thus constitute the selective advantage behind codon reassignments, which authenticates the "ambiguous decoding" hypothesis of genetic code evolution. Oxidative stress has shaped the mitochondrial genetic code.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Barrell BG, Bankier AT, Drouin J. A different genetic code in human mitochondria. Nature. 1979;282:189–194. - PubMed
-
- Knight RD, Freeland SJ, Landweber LF. Rewiring the keyboard: Evolvability of the genetic code. Nat Rev Genet. 2001;2:49–58. - PubMed
-
- Santos MA, Moura G, Massey SE, Tuite MF. Driving change: The evolution of alternative genetic codes. Trends Genet. 2004;20:95–102. - PubMed
-
- Crick FH. The origin of the genetic code. J Mol Biol. 1968;38:367–379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

