An internal domain of Exo70p is required for actin-independent localization and mediates assembly of specific exocyst components
- PMID: 18946089
- PMCID: PMC2613103
- DOI: 10.1091/mbc.e08-02-0157
An internal domain of Exo70p is required for actin-independent localization and mediates assembly of specific exocyst components
Abstract
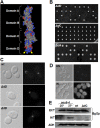
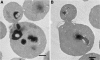
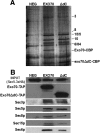
The exocyst consists of eight rod-shaped subunits that align in a side-by-side manner to tether secretory vesicles to the plasma membrane in preparation for fusion. Two subunits, Sec3p and Exo70p, localize to exocytic sites by an actin-independent pathway, whereas the other six ride on vesicles along actin cables. Here, we demonstrate that three of the four domains of Exo70p are essential for growth. The remaining domain, domain C, is not essential but when deleted, it leads to synthetic lethality with many secretory mutations, defects in exocyst assembly of exocyst components Sec5p and Sec6p, and loss of actin-independent localization. This is analogous to a deletion of the amino-terminal domain of Sec3p, which prevents an interaction with Cdc42p or Rho1p and blocks its actin-independent localization. The two mutations are synthetically lethal, even in the presence of high copy number suppressors that can bypass complete deletions of either single gene. Although domain C binds Rho3p, loss of the Exo70p-Rho3p interaction does not account for the synthetic lethal interactions or the exocyst assembly defects. The results suggest that either Exo70p or Sec3p must associate with the plasma membrane for the exocyst to function as a vesicle tether.
Figures





Similar articles
-
Bem1p contributes to secretory pathway polarization through a direct interaction with Exo70p.J Cell Biol. 2014 Oct 13;207(1):59-72. doi: 10.1083/jcb.201404122. J Cell Biol. 2014. PMID: 25313406 Free PMC article.
-
Membrane targeting of the yeast exocyst complex.Biochim Biophys Acta. 2015 Jul;1848(7):1481-9. doi: 10.1016/j.bbamem.2015.03.026. Epub 2015 Mar 30. Biochim Biophys Acta. 2015. PMID: 25838123
-
Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p.J Cell Biol. 2004 Dec 6;167(5):889-901. doi: 10.1083/jcb.200408124. J Cell Biol. 2004. PMID: 15583031 Free PMC article.
-
The exocyst complex in polarized exocytosis.Curr Opin Cell Biol. 2009 Aug;21(4):537-42. doi: 10.1016/j.ceb.2009.04.007. Epub 2009 May 25. Curr Opin Cell Biol. 2009. PMID: 19473826 Free PMC article. Review.
-
Exocytosis: the many masters of the exocyst.Curr Biol. 2002 Mar 19;12(6):R212-4. doi: 10.1016/s0960-9822(02)00753-4. Curr Biol. 2002. PMID: 11909549 Review.
Cited by
-
Emerging Roles of Exocyst Complex in Fungi: A Review.J Fungi (Basel). 2024 Aug 28;10(9):614. doi: 10.3390/jof10090614. J Fungi (Basel). 2024. PMID: 39330374 Free PMC article. Review.
-
The synaptobrevin homologue Snc2p recruits the exocyst to secretory vesicles by binding to Sec6p.J Cell Biol. 2013 Aug 5;202(3):509-26. doi: 10.1083/jcb.201211148. Epub 2013 Jul 29. J Cell Biol. 2013. PMID: 23897890 Free PMC article.
-
Sec6p anchors the assembled exocyst complex at sites of secretion.Mol Biol Cell. 2009 Feb;20(3):973-82. doi: 10.1091/mbc.e08-09-0968. Epub 2008 Dec 10. Mol Biol Cell. 2009. PMID: 19073882 Free PMC article.
-
Exocyst dynamics during vesicle tethering and fusion.Nat Commun. 2018 Dec 3;9(1):5140. doi: 10.1038/s41467-018-07467-5. Nat Commun. 2018. PMID: 30510181 Free PMC article.
-
Bem1p contributes to secretory pathway polarization through a direct interaction with Exo70p.J Cell Biol. 2014 Oct 13;207(1):59-72. doi: 10.1083/jcb.201404122. J Cell Biol. 2014. PMID: 25313406 Free PMC article.
References
-
- Abramoff M. D., Magelhaes P. J., Ram S. J. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42.
-
- Dong G., Hutagalung A., Fu C., Novick P., Reinisch K. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat. Struct. Mol. Biol. 2005;12:1094–1100. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

