Olfactory interference during inhibitory backward pairing in honey bees
- PMID: 18946512
- PMCID: PMC2568944
- DOI: 10.1371/journal.pone.0003513
Olfactory interference during inhibitory backward pairing in honey bees
Abstract
Background: Restrained worker honey bees are a valuable model for studying the behavioral and neural bases of olfactory plasticity. The proboscis extension response (PER; the proboscis is the mouthpart of honey bees) is released in response to sucrose stimulation. If sucrose stimulation is preceded one or a few times by an odor (forward pairing), the bee will form a memory for this association, and subsequent presentations of the odor alone are sufficient to elicit the PER. However, backward pairing between the two stimuli (sucrose, then odor) has not been studied to any great extent in bees, although the vertebrate literature indicates that it elicits a form of inhibitory plasticity.
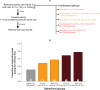
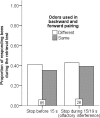
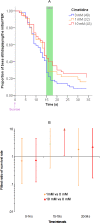
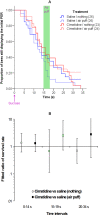
Methodology/principal findings: If hungry bees are fed with sucrose, they will release a long lasting PER; however, this PER can be interrupted if an odor is presented 15 seconds (but not 7 or 30 seconds) after the sucrose (backward pairing). We refer to this previously unreported process as olfactory interference. Bees receiving this 15 second backward pairing show reduced performance after a subsequent single forward pairing (excitatory conditioning) trial. Analysis of the results supported a relationship between olfactory interference and a form of backward pairing-induced inhibitory learning/memory. Injecting the drug cimetidine into the deutocerebrum impaired olfactory interference.
Conclusions/significance: Olfactory interference depends on the associative link between odor and PER, rather than between odor and sucrose. Furthermore, pairing an odor with sucrose can lead either to association of this odor to PER or to the inhibition of PER by this odor. Olfactory interference may provide insight into processes that gate how excitatory and inhibitory memories for odor-PER associations are formed.
Conflict of interest statement
Figures




References
-
- Menzel R, Giurfa M. Dimensions of cognition in an insect, the honeybee. Behav Cogn Neurosci Rev. 2006;5:24–40. - PubMed
-
- Menzel R, Leboulle G, Eisenhardt D. Small Brains, Bright Minds. Cell. 2006;124:237–239. - PubMed
-
- Menzel R, Giurfa M. Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci. 2001;5:62–71. - PubMed
-
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. - PubMed
-
- Krasne FB, Glanzman DL. What We can Learn from Invertebrate Learning. Annual Review of Psychology. 1995;46:585–624.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

