Period concatenation underlies interactions between gamma and beta rhythms in neocortex
- PMID: 18946516
- PMCID: PMC2525927
- DOI: 10.3389/neuro.03.001.2008
Period concatenation underlies interactions between gamma and beta rhythms in neocortex
Abstract
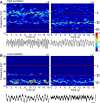
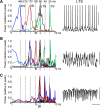
The neocortex generates rhythmic electrical activity over a frequency range covering many decades. Specific cognitive and motor states are associated with oscillations in discrete frequency bands within this range, but it is not known whether interactions and transitions between distinct frequencies are of functional importance. When coexpressed rhythms have frequencies that differ by a factor of two or more interactions can be seen in terms of phase synchronization. Larger frequency differences can result in interactions in the form of nesting of faster frequencies within slower ones by a process of amplitude modulation. It is not known how coexpressed rhythms, whose frequencies differ by less than a factor of two may interact. Here we show that two frequencies (gamma - 40 Hz and beta2 - 25 Hz), coexpressed in superficial and deep cortical laminae with low temporal interaction, can combine to generate a third frequency (beta1 - 15 Hz) showing strong temporal interaction. The process occurs via period concatenation, with basic rhythm-generating microcircuits underlying gamma and beta2 rhythms forming the building blocks of the beta1 rhythm by a process of addition. The mean ratio of adjacent frequency components was a constant - approximately the golden mean - which served to both minimize temporal interactions, and permit multiple transitions, between frequencies. The resulting temporal landscape may provide a framework for multiplexing - parallel information processing on multiple temporal scales.
Keywords: EEG; beta rhythm; gamma rhythm; inhibition; neocortex.
Figures




References
-
- Atela O., Gole C., Hotten S. (2002). A dynamical system for plant pattern formation: a rigorous analysis. J. Nonlinear Sci. 12, 641–67610.1007/s00332-002-0513-1 - DOI
LinkOut - more resources
Full Text Sources

