The effects of background noise on the neural responses to natural sounds in cat primary auditory cortex
- PMID: 18946525
- PMCID: PMC2525935
- DOI: 10.3389/neuro.10.003.2007
The effects of background noise on the neural responses to natural sounds in cat primary auditory cortex
Abstract
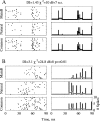
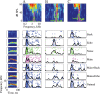
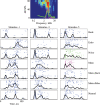
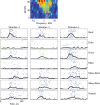
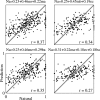
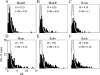
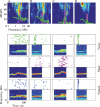
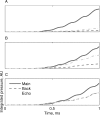
Animal vocalizations in natural settings are invariably accompanied by an acoustic background with a complex statistical structure. We have previously demonstrated that neuronal responses in primary auditory cortex of halothane-anesthetized cats depend strongly on the natural background. Here, we study in detail the neuronal responses to the background sounds and their relationships to the responses to the foreground sounds. Natural bird chirps as well as modifications of these chirps were used. The chirps were decomposed into three components: the clean chirps, their echoes, and the background noise. The last two were weaker than the clean chirp by 13 and 29 dB on average respectively. The test stimuli consisted of the full natural stimulus, the three basic components, and their three pairwise combinations. When the level of the background components (echoes and background noise) presented alone was sufficiently loud to evoke neuronal activity, these background components had an unexpectedly strong effect on the responses of the neurons to the main bird chirp. In particular, the responses to the original chirps were more similar on average to the responses evoked by the two background components than to the responses evoked by the clean chirp, both in terms of the evoked spike count and in terms of the temporal pattern of the responses. These results suggest that some of the neurons responded specifically to the acoustic background even when presented together with the substantially louder main chirp, and may imply that neurons in A1 already participate in auditory source segregation.
Keywords: auditory cortex; cats; electrophysiology; natural sounds; single neurons.
Figures


References
-
- Bizley J. K., Nodal F. R., Nelken I., King A. J. (2005). Functional organization of Ferret auditory cortex. Cereb. Cortex. - PubMed
-
- Creutzfeldt O., Hellweg F. C., Schreiner C. (1980). Thalamocortical transformation of responses to complex auditory stimuli. Exp. Brain Res. 39, 87–104 - PubMed
-
- Famoye F. (2000). Goodness-of-fit tests for generalized logarithmic series distribution. Comput. Stat. Data Analysis 33, 59–67
-
- Gehr D. D., Komiya H., Eggermont J. J. (2000). Neuronal responses in cat primary auditory cortex to natural and altered species-specific calls. Hear. Res. 150, 27–42 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

