Estimating firing rates from calcium signals in locust projection neurons in vivo
- PMID: 18946544
- PMCID: PMC2526277
- DOI: 10.3389/neuro.04.002.2007
Estimating firing rates from calcium signals in locust projection neurons in vivo
Abstract
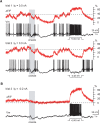
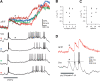
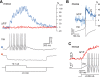
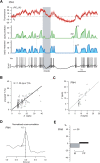
Combining intracellular electrophysiology and multi-photon calcium imaging in vivo, we studied the relationship between calcium signals (sampled at 500-750 Hz) and spike output in principal neurons in the locust antennal lobe. Our goal was to determine whether the firing rate of individual neurons can be estimated in vivo with calcium imaging and, if so, to measure directly the accuracy and resolution of our estimates. Using the calcium indicator Oregon Green BAPTA-1, we describe a simple method to reconstruct firing rates from dendritic calcium signals with 80-90% accuracy and 50 ms temporal resolution.
Keywords: Oregon Green BAPTA-1; calcium imaging; electrophysiology; locust; olfaction; projection neuron; spiking activity; two-photon microscopy.
Figures




References
-
- Bicker G. (1999). Histochemistry of classical neurotransmitters in antennal lobes and mushroom bodies of the honeybee. Microsc. Res. Tech. 45, 174–183 - PubMed
-
- Brustein E., Marandi N., Kovalchuk Y., Drapeau P., Konnerth A. (2003). “In vivo” monitoring of neuronal network activity in zebrafish by two-photon Ca(2+) imaging. Pflugers Arch. 446, 766–773 - PubMed
-
- Buck L., Axel R. (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187 - PubMed
-
- Buzsaki G. (2004). Large-scale recording of neuronal ensembles. Nat. Neurosci. 7, 446–451 - PubMed
-
- Carlsson M. A., Knusel P., Verschure P. F., Hansson B. S. (2005). Spatio-temporal Ca2+ dynamics of moth olfactory projection neurones. Eur. J. Neurosci. 22, 647–657 - PubMed
LinkOut - more resources
Full Text Sources

