Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection
- PMID: 18947414
- PMCID: PMC2588578
- DOI: 10.1186/1742-2094-5-48
Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection
Abstract
Background: Worldwide, approximately two billion people are chronically infected with Toxoplasma gondii with largely unknown consequences.
Methods: To better understand long-term effects and pathogenesis of this common, persistent brain infection, mice were infected at a time in human years equivalent to early to mid adulthood and studied 5-12 months later. Appearance, behavior, neurologic function and brain MRIs were studied. Additional analyses of pathogenesis included: correlation of brain weight and neurologic findings; histopathology focusing on brain regions; full genome microarrays; immunohistochemistry characterizing inflammatory cells; determination of presence of tachyzoites and bradyzoites; electron microscopy; and study of markers of inflammation in serum. Histopathology in genetically resistant mice and cytokine and NRAMP knockout mice, effects of inoculation of isolated parasites, and treatment with sulfadiazine or alphaPD1 ligand were studied.
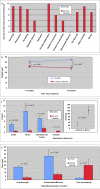
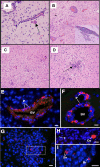
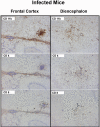
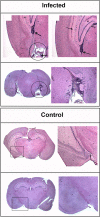
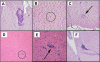
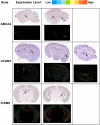
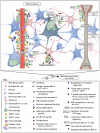
Results: Twelve months after infection, a time equivalent to middle to early elderly ages, mice had behavioral and neurological deficits, and brain MRIs showed mild to moderate ventricular dilatation. Lower brain weight correlated with greater magnitude of neurologic abnormalities and inflammation. Full genome microarrays of brains reflected inflammation causing neuronal damage (Gfap), effects on host cell protein processing (ubiquitin ligase), synapse remodeling (Complement 1q), and also increased expression of PD-1L (a ligand that allows persistent LCMV brain infection) and CD 36 (a fatty acid translocase and oxidized LDL receptor that mediates innate immune response to beta amyloid which is associated with pro-inflammation in Alzheimer's disease). Immunostaining detected no inflammation around intra-neuronal cysts, practically no free tachyzoites, and only rare bradyzoites. Nonetheless, there were perivascular, leptomeningeal inflammatory cells, particularly contiguous to the aqueduct of Sylvius and hippocampus, CD4+ and CD8+ T cells, and activated microglia in perivascular areas and brain parenchyma. Genetically resistant, chronically infected mice had substantially less inflammation.
Conclusion: In outbred mice, chronic, adult acquired T. gondii infection causes neurologic and behavioral abnormalities secondary to inflammation and loss of brain parenchyma. Perivascular inflammation is prominent particularly contiguous to the aqueduct of Sylvius and hippocampus. Even resistant mice have perivascular inflammation. This mouse model of chronic T. gondii infection raises questions of whether persistence of this parasite in brain can cause inflammation or neurodegeneration in genetically susceptible hosts.
Figures



References
-
- Boyer K, Marcinak J, McLeod R. Toxoplasma gondii (Toxoplasmosis) In: Long S, Pickering LK, Prober CG, editor. Principles and Practice of Pediatric Infectious Diseases. 3. New York: Churchill Livingstone; 2007.
-
- Flegr J, Preiss M, Klose J, Havlicek J, Vitakova M, Kodym P. Decreased level of psychobiological factor novelty seeking and lower intelligence in men latently infected with the protozoan parasite Toxoplasma gondii Dopamine, a missing link between schizophrenia and toxoplasmosis? Biol Psychol. 2003;63:253–268. - PubMed
-
- Palmer BS. Meta-analysis of three case controlled studies and an ecological study into the link between cryptogenic epilepsy and chronic toxoplasmosis infection. Seizure. 2007;16:657–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

