RACK1 genes regulate plant development with unequal genetic redundancy in Arabidopsis
- PMID: 18947417
- PMCID: PMC2577656
- DOI: 10.1186/1471-2229-8-108
RACK1 genes regulate plant development with unequal genetic redundancy in Arabidopsis
Abstract
Background: RACK1 is a versatile scaffold protein in mammals, regulating diverse developmental processes. Unlike in non-plant organisms where RACK1 is encoded by a single gene, Arabidopsis genome contains three RACK1 homologous genes, designated as RACK1A, RACK1B and RACK1C, respectively. Previous studies indicated that the loss-of-function alleles of RACK1A displayed multiple defects in plant development. However, the functions of RACK1B and RACK1C remain elusive. Further, the relationships between three RACK1 homologous genes are unknown.
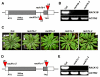
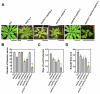
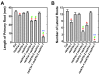
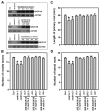
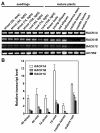
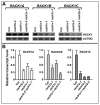
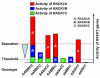
Results: We isolated mutant alleles with loss-of-function mutations in RACK1B and RACK1C, and examined the impact of these mutations on plant development. We found that unlike in RACK1A, loss-of-function mutations in RACK1B or RACK1C do not confer apparent defects in plant development, including rosette leaf production and root development. Analyses of rack1a, rack1b and rack1c double and triple mutants, however, revealed that rack1b and rack1c can enhance the rack1a mutant's developmental defects, and an extreme developmental defect and lethality were observed in rack1a rack1b rack1c triple mutant. Complementation studies indicated that RACK1B and RACK1C are in principle functionally equivalent to RACK1A. Gene expression studies indicated that three RACK1 genes display similar expression patterns but are expressed at different levels. Further, RACK1 genes positively regulate each other's expression.
Conclusion: These results suggested that RACK1 genes are critical regulators of plant development and that RACK1 genes function in an unequally redundant manner. Both the difference in RACK1 gene expression level and the cross-regulation are likely the molecular determinants of their unequal genetic redundancy.
Figures

Similar articles
-
Dissection of the relationship between RACK1 and heterotrimeric G-proteins in Arabidopsis.Plant Cell Physiol. 2009 Sep;50(9):1681-94. doi: 10.1093/pcp/pcp113. Epub 2009 Aug 3. Plant Cell Physiol. 2009. PMID: 19651700
-
ASYMMETRIC LEAVES1, an Arabidopsis gene that is involved in the control of cell differentiation in leaves.Planta. 2002 Mar;214(5):694-702. doi: 10.1007/s004250100673. Epub 2001 Nov 10. Planta. 2002. PMID: 11882937
-
Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves.Plant Cell Physiol. 2009 Jun;50(6):1019-31. doi: 10.1093/pcp/pcp068. Epub 2009 May 12. Plant Cell Physiol. 2009. PMID: 19435754
-
Unequal genetic redundancies in Arabidopsis--a neglected phenomenon?Trends Plant Sci. 2006 Oct;11(10):492-8. doi: 10.1016/j.tplants.2006.08.005. Epub 2006 Sep 1. Trends Plant Sci. 2006. PMID: 16949326 Review.
-
Transcription factors regulating leaf senescence in Arabidopsis thaliana.Plant Biol (Stuttg). 2008 Sep;10 Suppl 1:63-75. doi: 10.1111/j.1438-8677.2008.00088.x. Plant Biol (Stuttg). 2008. PMID: 18721312 Review.
Cited by
-
Phyllotaxis: from classical knowledge to molecular genetics.J Plant Res. 2021 May;134(3):373-401. doi: 10.1007/s10265-020-01247-3. Epub 2021 Feb 7. J Plant Res. 2021. PMID: 33550488 Review.
-
Involvement of Arabidopsis RACK1 in protein translation and its regulation by abscisic acid.Plant Physiol. 2011 Jan;155(1):370-83. doi: 10.1104/pp.110.160663. Epub 2010 Nov 19. Plant Physiol. 2011. PMID: 21098678 Free PMC article.
-
Proteomic profiling of endothelial invasion revealed receptor for activated C kinase 1 (RACK1) complexed with vimentin to regulate focal adhesion kinase (FAK).J Biol Chem. 2013 Oct 18;288(42):30720-30733. doi: 10.1074/jbc.M113.512467. Epub 2013 Sep 4. J Biol Chem. 2013. PMID: 24005669 Free PMC article.
-
RACK1, A multifaceted scaffolding protein: Structure and function.Cell Commun Signal. 2011 Oct 6;9:22. doi: 10.1186/1478-811X-9-22. Cell Commun Signal. 2011. PMID: 21978545 Free PMC article.
-
The turnover of ABI5 by scaffold proteins to attenuate ABA signaling.J Exp Bot. 2024 Jul 10;75(13):3749-3753. doi: 10.1093/jxb/erae226. J Exp Bot. 2024. PMID: 38982747 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

