Variation in the definition of clinical target volumes for pelvic nodal conformal radiation therapy for prostate cancer
- PMID: 18947941
- PMCID: PMC2905162
- DOI: 10.1016/j.ijrobp.2008.08.003
Variation in the definition of clinical target volumes for pelvic nodal conformal radiation therapy for prostate cancer
Abstract
Purpose: We conducted a comparative study of clinical target volume (CTV) definition of pelvic lymph nodes by multiple genitourinary (GU) radiation oncologists looking at the levels of discrepancies amongst this group.
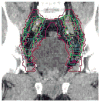
Methods and materials: Pelvic computed tomography (CT) scans from 2 men were distributed to 14 Radiation Therapy Oncology Group GU radiation oncologists with instructions to define CTVs for the iliac and presacral lymph nodes. The CT data with contours were then returned for analysis. In addition, a questionnaire was completed that described the physicians' method for target volume definition.
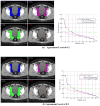
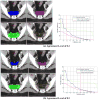
Results: Significant variation in the definition of the iliac and presacral CTVs was seen among the physicians. The minimum, maximum, mean (SD) iliac volumes (mL) were 81.8, 876.6, 337.6 +/- 203 for case 1 and 60.3, 627.7, 251.8 +/- 159.3 for case 2. The volume of 100% agreement was 30.6 and 17.4 for case 1 and 2 and the volume of the union of all contours was 1,012.0 and 807.4 for case 1 and 2, respectively. The overall agreement was judged to be moderate in both cases (kappa = 0.53 (p < 0.0001) and kappa = 0.48 (p < 0.0001). There was no volume of 100% agreement for either of the two presacral volumes. These variations were confirmed in the responses to the associated questionnaire.
Conclusions: Significant disagreement exists in the definition of the CTV for pelvic nodal radiation therapy among GU radiation oncology specialists. A consensus needs to be developed so as to accurately assess the merit and safety of such treatment.
Conflict of interest statement
Conflict of Interest: None
Figures



References
-
- Pilepich M, Winter K, Lawton C, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma long term results of phase III RTOG 85–31. Int J Rad Onc Biol Phys. 2005;61(5):1285–1290. - PubMed
-
- Roach M, III, Bae K, Speight J, et al. Short Term Neoadjuvant Androgen Deprivation Therapy and External Beam Radiotherapy for Locally Advanced Prostate Cancer: Long Term Results of RTOG 8610 a Phase III Prospective Randomized Trial. Journal of Clinical Oncology. 2008;26(4):585–591. - PubMed
-
- Bolla M, Collette L, Blank L, et al. Long term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study, a phase III randomized trial) Lancet. 2002;360:103–108. - PubMed
-
- Horwitz E, Bae K, Hanks G, Porter A, Grignon D, Brereton H, Venkatesan V, Lawton C, et al. Ten-year follow-up of RTOG 92–02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Onc. 2008 May 20;26(15):2497–2504. - PubMed
-
- Lawton C, DeSilvio M, Roach M, III, et al. An Update of the Phase III Trial Comparing Whole-Pelvic (WP) to Prostate Only (PO) Radiotherapy and Neoadjuvant to Adjuvant Total Androgen Suppression (TAS): Updated Analysis of RTOG 94–13, with Emphasis on Unexpected Hormone/Radiation Interactions. Int J Rad Onc Biol & Phys. 2007;69(3):646–655. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

