Simultaneous quantitation of 17alpha-hydroxyprogesterone caproate, 17alpha-hydroxyprogesterone and progesterone in human plasma using high-performance liquid chromatography-mass spectrometry (HPLC-MS/MS)
- PMID: 18947956
- PMCID: PMC4138984
- DOI: 10.1016/j.jpba.2008.08.024
Simultaneous quantitation of 17alpha-hydroxyprogesterone caproate, 17alpha-hydroxyprogesterone and progesterone in human plasma using high-performance liquid chromatography-mass spectrometry (HPLC-MS/MS)
Abstract
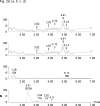
A sensitive and specific assay method for the simultaneous quantitation of 17alpha-hydroxyprogesterone caproate (17-OHPC), 17alpha-hydroxyprogesterone (17-OHP), and progesterone (P) in human plasma using high-performance liquid chromatography and tandem mass spectrometry (LC-MS/MS) was developed and validated. Plasma samples were processed by a solid phase extraction (SPE) procedure using Oasis((R)) HLB extraction cartridge prior to chromatography. Medroxyprogestrone acetate (MPA) was used as the internal standard. The compounds were separated using Waters C18 Symmetry analytical column (3.5 microm, 2.1 mm x 50 mm) using a gradient elusion with a mobile phase consisting of 5% methanol in water [A] and methanol [B], with ammonium acetate (2mM) and formic acid (0.1%) being added to both [A] and [B], at a flow rate 0.3 ml/min. The retention times for 17-OHPC, 17-OHP, P and MPA were 4.5, 1.5, 2.5 and 2.2 min, respectively, with a total run time of 7 min. The analytes were detected by a Micromass Quattro Micro triple quadrupole mass spectrometer in positive electron spray ionization (ESI) mode using multiple reaction monitoring (MRM). The extracted ions monitored following MRM transitions were m/z 429.10-->313.10 for 17-OHPC, m/z 331.17-->97.00 for 17-OHP, m/z 315.15-->109.00 for P and m/z 387.15-->327.25 for MPA (IS). The assay was linear over the range 1-200 ng/ml for 17-OHPC and 17-OHP, and 2-400 ng/ml for P, when 0.4 ml of plasma was used in the extraction. The overall intra- and inter-day assay variation was <15%. No significant variation in the concentration of 17-OHPC, 17-OHP or P was observed with different sample processing and/or storage conditions. This method is simple, allows easy, accurate and reproducible measurement of 17-OHPC, 17-OHP and P simultaneously in human plasma, and is used to evaluate the pharmacokinetics of 17-OHPC in pregnant subjects.
Figures
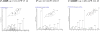



References
-
- Paneth NS. Future Child. 1995;5:19–34. - PubMed
-
- Mattison DR, Damus K, Fiore E, Petrini J, Alter C. Paediatr Perinat Epidemiol. 2001;2:7–16. - PubMed
-
- Meis PJ. Obstet Gynecol. 2005;105:1128–1135. - PubMed
-
- Petrini JR, Callaghan WM, Klebanoff M, Green NS, Lackritz EM, Howse JL, Schwarz RH, Damus K. Obstet Gynecol. 2005;105:267–272. - PubMed
-
- Sanchez-Ramos L, Kaunitz AM, Delke I. Obstet Gynecol. 2005;105:273–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

