The astrocyte odyssey
- PMID: 18948166
- PMCID: PMC2613184
- DOI: 10.1016/j.pneurobio.2008.09.015
The astrocyte odyssey
Abstract
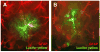
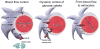
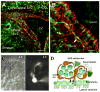
Neurons have long held the spotlight as the central players of the nervous system, but we must remember that we have equal numbers of astrocytes and neurons in the brain. Are these cells only filling up the space and passively nurturing the neurons, or do they also contribute to information transfer and processing? After several years of intense research since the pioneer discovery of astrocytic calcium waves and glutamate release onto neurons in vitro, the neuronal-glial studies have answered many questions thanks to technological advances. However, the definitive in vivo role of astrocytes remains to be addressed. In addition, it is becoming clear that diverse populations of astrocytes coexist with different molecular identities and specialized functions adjusted to their microenvironment, but do they all belong to the umbrella family of astrocytes? One population of astrocytes takes on a new function by displaying both support cell and stem cell characteristics in the neurogenic niches. Here, we define characteristics that classify a cell as an astrocyte under physiological conditions. We will also discuss the well-established and emerging functions of astrocytes with an emphasis on their roles on neuronal activity and as neural stem cells in adult neurogenic zones.
Figures



References
-
- Abbott NJ, Revest PA. Control of brain endothelial permeability. Cerebrovasc Brain Metab Rev. 1991;3:39–72. - PubMed
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 2006;276:91–103. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

