Structural insights into ABC transporter mechanism
- PMID: 18948194
- PMCID: PMC2643341
- DOI: 10.1016/j.sbi.2008.09.007
Structural insights into ABC transporter mechanism
Abstract
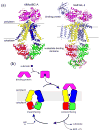
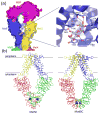
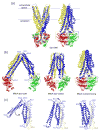
ATP-binding cassette (ABC) transporters utilize the energy from ATP hydrolysis to transport substances across the membrane. In recent years, crystal structures of several ABC transporters have become available. These structures show that both importers and exporters oscillate between two conformations: an inward-facing conformation with the substrate translocation pathway open to the cytoplasm and an outward-facing conformation with the translocation pathway facing the opposite side of the membrane. In this review, conformational differences found in the structures of homologous ABC transporters are analyzed to understand how alternating-access is achieved. It appears that rigid-body rotations of the transmembrane subunits, coinciding with the opening and closing of the nucleotide-binding subunits, couples ATP hydrolysis to substrate translocation.
Conflict of interest statement
The authors declare no conflicts of interest
Figures
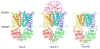
References
-
- Biemans-Oldehinkel E, Doeven MK, Poolman B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 2006;580:1023–1035. - PubMed
-
- Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412–418. - PubMed
-
- Dawson RJ, Hollenstein K, Locher KP. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol Microbiol. 2007;65:250–257. - PubMed
-
- Davidson AL, Maloney PC. ABC transporters: how small machines do a big job. Trends Microbiol. 2007;15:448–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

