A novel protein domain induces high affinity selenocysteine insertion sequence binding and elongation factor recruitment
- PMID: 18948268
- PMCID: PMC3073842
- DOI: 10.1074/jbc.M806008200
A novel protein domain induces high affinity selenocysteine insertion sequence binding and elongation factor recruitment
Abstract
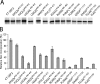
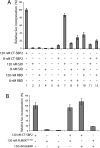
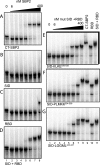
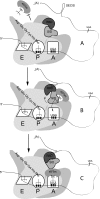
Selenocysteine (Sec) is incorporated at UGA codons in mRNAs possessing a Sec insertion sequence (SECIS) element in their 3'-untranslated region. At least three additional factors are necessary for Sec incorporation: SECIS-binding protein 2 (SBP2), Sec-tRNA(Sec), and a Sec-specific translation elongation factor (eEFSec). The C-terminal half of SBP2 is sufficient to promote Sec incorporation in vitro, which is carried out by the concerted action of a novel Sec incorporation domain and an L7Ae RNA-binding domain. Using alanine scanning mutagenesis, we show that two distinct regions of the Sec incorporation domain are required for Sec incorporation. Physical separation of the Sec incorporation and RNA-binding domains revealed that they are able to function in trans and established a novel role of the Sec incorporation domain in promoting SECIS and eEFSec binding to the SBP2 RNA-binding domain. We propose a model in which SECIS binding induces a conformational change in SBP2 that recruits eEFSec, which in concert with the Sec incorporation domain gains access to the ribosomal A site.
Figures





References
-
- Zavacki, A. M., Mansell, J. B., Chung, M., Klimovitsky, B., Harney, J. W., and Berry, M. J. (2003) Mol Cell 11, 773-781 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

