Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice
- PMID: 18948273
- PMCID: PMC2639107
- DOI: 10.1093/cvr/cvn286
Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice
Abstract
Aims: The risk of adverse cardiovascular events in humans is increased with chronic use of cyclooxygenase-2 (COX-2) inhibitors. However, the role of COX-2 in animal models of cardiovascular disease has been controversial. In humans and animal models, cardiovascular disease is increased by bacterial infection of the supporting tissue of the teeth, a condition known as periodontal disease. Periodontal disease may result in chronic exposure to pro-inflammatory mediators, such as bacterial lipopolysaccharide (LPS), thereby producing a systemic inflammatory response. The current study examined the role of COX-2 in atherosclerosis induced by LPS derived from the periodontal disease pathogen Porphyromonas gingivalis (P. gingivalis).
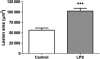
Methods and results: Porphyromonas gingivalis LPS was administered by chronic infusion for 28 days and atherosclerosis development was examined in the aortic root of ApoE (apolipoprotein E)-deficient mice. The extent of atherosclerosis was compared between mice receiving control diet or diet containing the COX-2 inhibitor celecoxib. The role of COX-2 in P. gingivalis LPS-induced inflammatory cell activation was examined in peritoneal macrophages. Porphyromonas gingivalis LPS infusion significantly increased atherosclerosis development. In mice infused with P. gingivalis LPS, administration of the COX-2 inhibitor celecoxib further increased the extent of atherosclerotic lesion area. In peritoneal macrophages, P. gingivalis LPS increased the expression of COX-2 mRNA (messenger ribonucleic acid) and the production of prostaglandin (PG) E(2) (PGE(2)), the latter of which was inhibited by celecoxib. Porphyromonas gingivalis LPS-induced expression of tumour necrosis factor alpha (TNFalpha) was enhanced by inactivation of COX-2 and was attenuated by treatment with PGE(2).
Conclusion: The inhibition of COX-2-derived PGE(2) may enhance P. gingivalis LPS-induced atherosclerosis by increasing macrophage production of TNFalpha.
Figures




Similar articles
-
Neuroprotective effects of cyclooxygenase-2 inhibitor celecoxib against toxicity of LPS-stimulated macrophages toward motor neurons.Acta Pharmacol Sin. 2005 Aug;26(8):952-8. doi: 10.1111/j.1745-7254.2005.00136.x. Acta Pharmacol Sin. 2005. PMID: 16038627
-
The cyclooxygenase 2 genetic variant -765G>C does not modulate the effects of celecoxib on prostaglandin E2 production.Clin Pharmacol Ther. 2006 Dec;80(6):621-32. doi: 10.1016/j.clpt.2006.08.021. Clin Pharmacol Ther. 2006. PMID: 17178263 Clinical Trial.
-
Cyclooxygenase-2 promotes early atherosclerotic lesion formation in ApoE-deficient and C57BL/6 mice.J Mol Cell Cardiol. 2005 Sep;39(3):443-52. doi: 10.1016/j.yjmcc.2005.06.011. J Mol Cell Cardiol. 2005. PMID: 16040051
-
Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis.Front Biosci. 2008 Jan 1;13:2041-59. doi: 10.2741/2822. Front Biosci. 2008. PMID: 17981690 Review.
-
Celecoxib for the treatment of atherosclerosis.Expert Opin Investig Drugs. 2016;25(5):619-33. doi: 10.1517/13543784.2016.1161756. Epub 2016 Mar 21. Expert Opin Investig Drugs. 2016. PMID: 26940257 Review.
Cited by
-
A 4-trifluoromethyl analogue of celecoxib inhibits arthritis by suppressing innate immune cell activation.Arthritis Res Ther. 2012 Jan 17;14(1):R9. doi: 10.1186/ar3683. Arthritis Res Ther. 2012. PMID: 22251404 Free PMC article.
-
Inflammation and Vascular Effects after Repeated Intratracheal Instillations of Carbon Black and Lipopolysaccharide.PLoS One. 2016 Aug 29;11(8):e0160731. doi: 10.1371/journal.pone.0160731. eCollection 2016. PLoS One. 2016. PMID: 27571356 Free PMC article.
-
Particle-induced pulmonary acute phase response correlates with neutrophil influx linking inhaled particles and cardiovascular risk.PLoS One. 2013 Jul 24;8(7):e69020. doi: 10.1371/journal.pone.0069020. Print 2013. PLoS One. 2013. PMID: 23894396 Free PMC article.
-
Eicosanoids and other oxylipins in liver injury, inflammation and liver cancer development.Front Physiol. 2023 Feb 2;14:1098467. doi: 10.3389/fphys.2023.1098467. eCollection 2023. Front Physiol. 2023. PMID: 36818443 Free PMC article. Review.
-
Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution.Nat Metab. 2022 Apr;4(4):444-457. doi: 10.1038/s42255-022-00551-7. Epub 2022 Mar 31. Nat Metab. 2022. PMID: 35361955 Free PMC article.
References
-
- Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. - PubMed
-
- Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. - PubMed
-
- Pussinen PJ, Tuomisto K, Jousilahti P, Havulinna AS, Sundvall J, Salomaa V. Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arterioscler Thromb Vasc Biol. 2007;27:1433–1439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

