Molecular characterization of nucleocytosolic O-GlcNAc transferases of Giardia lamblia and Cryptosporidium parvum
- PMID: 18948359
- PMCID: PMC2733775
- DOI: 10.1093/glycob/cwn107
Molecular characterization of nucleocytosolic O-GlcNAc transferases of Giardia lamblia and Cryptosporidium parvum
Abstract
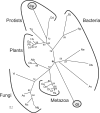
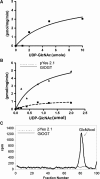
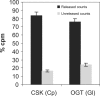
O-Linked N-acetylglucosaminyltransferase (OGT) catalyzes the transfer of a single GlcNAc to the Ser or Thr of nucleocytoplasmic proteins. OGT activity, which may compete with that of kinases, is involved in signaling in animals and plants, and abnormalities in OGT activities have been associated with type 2 diabetes. Here, we show that ogt genes that predict enzymes with characteristic tetratricopeptide repeats and a spindly domain are present in some protists (Giardia, Cryptosporidium, Toxoplasma, and Dictyostelium) but are absent from the majority of protists examined (e.g., Plasmodium, Trypanosoma, Entamoeba, and Trichomonas). Similarly, ogt genes are present in some fungi but are absent from numerous other fungi, suggesting that secondary loss is an important contributor to the evolution of ogt genes. Nucleocytosolic extracts of Giardia and Cryptosporidium show OGT activity, and recombinant Giardia and Cryptosporidium OGTs are active in yeast and bacteria, respectively. These results suggest the possibility that O-GlcNAc modification of nucleocytosolic proteins also has function(s) in simple eukaryotes.
Figures

References
-
- Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. - PubMed
-
- Cheng X, Cole RN, Zaia J, Hart GW. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry. 2000;39:11609–11620. - PubMed
-
- de Koning AP, Brinkman FS, Jones SJ, Keeling PJ. Lateral gene transfer and metabolic adaptation in the human parasite Trichomonas vaginalis. Mol Biol Evol. 2000;17:1769–1773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

