The Mn1 transcription factor acts upstream of Tbx22 and preferentially regulates posterior palate growth in mice
- PMID: 18948418
- PMCID: PMC2586179
- DOI: 10.1242/dev.025304
The Mn1 transcription factor acts upstream of Tbx22 and preferentially regulates posterior palate growth in mice
Abstract
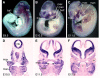
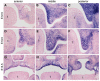
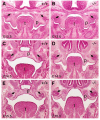
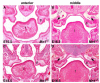
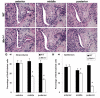
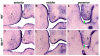
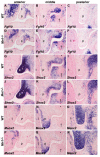
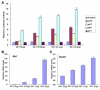
The mammalian secondary palate exhibits morphological, pathological and molecular heterogeneity along the anteroposterior axis. Although the cell proliferation rates are similar in the anterior and posterior regions during palatal outgrowth, previous studies have identified several signaling pathways and transcription factors that specifically regulate the growth of the anterior palate. By contrast, no factor has been shown to preferentially regulate posterior palatal growth. Here, we show that mice lacking the transcription factor Mn1 have defects in posterior but not anterior palatal growth. We show that Mn1 mRNA exhibits differential expression along the anteroposterior axis of the developing secondary palate, with preferential expression in the middle and posterior regions during palatal outgrowth. Extensive analyses of palatal gene expression in wild-type and Mn1(-/-) mutant mice identified Tbx22, the mouse homolog of the human X-linked cleft palate gene, as a putative downstream target of Mn1 transcriptional activation. Tbx22 exhibits a similar pattern of expression with that of Mn1 along the anteroposterior axis of the developing palatal shelves and its expression is specifically downregulated in Mn1(-/-) mutants. Moreover, we show that Mn1 activated reporter gene expression driven by either the human or mouse Tbx22 gene promoters in co-transfected NIH3T3 cells. Overexpression of Mn1 in NIH3T3 cells also increased endogenous Tbx22 mRNA expression in a dose-dependent manner. These data indicate that Mn1 and Tbx22 function in a novel molecular pathway regulating mammalian palate development.
Figures


References
-
- Alappat SR, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, Yamada G, Chen Y. The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev. Biol. 2005;277:102–113. - PubMed
-
- Braybrook C, Doudney K, Marçano AC, Arnason A, Bjornsson A, Patton MA, Goodfellow PJ, Moore GE, Stanier P. The T-box transcription factor gene TBX22 is mutated in X-linked cleft palate and ankyloglossia. Nat. Genet. 2001;29:179–183. - PubMed
-
- Braybrook C, Lisgo S, Doudney K, Henderson D, Marçano AC, Strachan T, Patton MA, Villard L, Moore GE, Stanier P, Lindsay S. Craniofacial expression of human and murine TBX22 correlates with the cleft palate and ankyloglossia phenotype observed in CPX patients. Hum. Mol. Genet. 2002;11:2793–804. - PubMed
-
- Buijs A, Sherr S, van Baal S, van Bezouw S, van der Plas D, van Kessel A. Geurts, Riegman P, Deprez R. Lekanne, Zwarthoff E, Hagemeijer A. Translocation (12;22) (p13;q11) in myeloproliferative disorders results in fusion of the ETS-like TEL gene on 12p13 to the MN1 gene on 22q11. Oncogene. 1995;10:1511–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

