Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55
- PMID: 18948538
- PMCID: PMC2720046
- DOI: 10.1126/science.1162042
Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55
Abstract
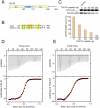
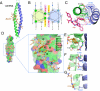
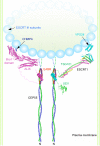
The ESCRT (endosomal sorting complex required for transport) machinery is required for the scission of membrane necks in processes including the budding of HIV-1 and cytokinesis. An essential step in cytokinesis is recruitment of the ESCRT-I complex and the ESCRT-associated protein ALIX to the midbody (the structure that tethers two daughter cells) by the protein CEP55. Biochemical experiments show that peptides from ALIX and the ESCRT-I subunit TSG101 compete for binding to the ESCRT and ALIX-binding region (EABR) of CEP55. We solved the crystal structure of EABR bound to an ALIX peptide at a resolution of 2.0 angstroms. The structure shows that EABR forms an aberrant dimeric parallel coiled coil. Bulky and charged residues at the interface of the two central heptad repeats create asymmetry and a single binding site for an ALIX or TSG101 peptide. Both ALIX and ESCRT-I are required for cytokinesis, which suggests that multiple CEP55 dimers are required for function.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

