Effect of raloxifene on stroke and venous thromboembolism according to subgroups in postmenopausal women at increased risk of coronary heart disease
- PMID: 18948611
- PMCID: PMC3559135
- DOI: 10.1161/STROKEAHA.108.518621
Effect of raloxifene on stroke and venous thromboembolism according to subgroups in postmenopausal women at increased risk of coronary heart disease
Abstract
Background and purpose: Raloxifene, a selective estrogen receptor modulator, reduces risk of invasive breast cancer and osteoporosis, but the effect on risk for stroke and venous thromboembolism in different patient subgroups is not established. The purpose of this analysis was to evaluate the effect of raloxifene on the incidence of all strokes, stroke deaths, and venous thromboembolic events according to participant subgroups.
Methods: This was a secondary end point analysis of an international, randomized, placebo-controlled clinical trial of 10 101 postmenopausal women with or at increased risk of coronary heart disease followed a median of 5.6 years. Strokes, venous thromboembolic events, and deaths were adjudicated by expert centralized committees. Strokes were categorized as ischemic, hemorrhagic, or undetermined and venous thromboembolic events were subclassified.
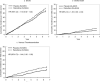
Results: The incidences of all strokes did not differ between raloxifene (incidence rate per 100 woman-years=0.95) and placebo (incidence rate=0.86) treatment groups (P=0.30). In women assigned raloxifene versus placebo, there was a higher incidence of fatal strokes (incidence rates=0.22 and 0.15, respectively, P=0.0499) and venous thromboembolic events (incidence rates=0.39 and 0.27, respectively, P=0.02). No significant subgroup interactions were found except that there was a higher incidence of stroke associated with raloxifene use among current smokers.
Conclusions: In postmenopausal women at increased risk for coronary events, the incidences of venous thromboembolism and fatal stroke but not all strokes were higher in those assigned raloxifene versus placebo. Raloxifene's effect did not differ across subgroups, except that the risk of stroke differed by smoking status. Treatment decisions about raloxifene should be based on a balance of projected absolute risks and benefits.
Figures


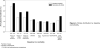

References
-
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady G, Kornitzer M, McNabb MA, Wenger NK. Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–237. - PubMed
-
- Stefanick ML. Risk–benefit profiles of raloxifene for women. N Engl J Med. 2006;355:190–192. - PubMed
-
- Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, Vittinghoff E, Hulley S. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132:689–696. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
-
- Grady D, Ettinger B, Moscarelli E, Plouffe L, Jr, Sarkar S, Ciaccia A, Cummings S, Multiple Outcomes of Raloxifene Evaluation Investigators Safety and adverse effects associated with raloxifene: Multiple outcomes of raloxifene evaluation. Obstet Gynecol. 2004;104:837–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

