Brothers and sisters: molecular insights into arterial-venous heterogeneity
- PMID: 18948631
- PMCID: PMC2760069
- DOI: 10.1161/CIRCRESAHA.108.184937
Brothers and sisters: molecular insights into arterial-venous heterogeneity
Abstract
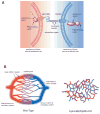
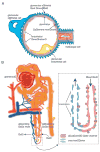
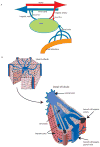
The molecular differences between arteries and veins are genetically predetermined and are evident even before the first embryonic heart beat. Although ephrinB2 and EphB4 are expressed in cells that will ultimately differentiate into arteries and veins, respectively, many other genes have been shown to play a significant role in cell fate determination. The expression patterns of ephrinB2 and EphB4 are restricted to arterial-venous boundaries, and Eph/ephrin signaling provides repulsive cues at arterial-venous boundaries that are thought to prevent intermixing of arterial- and venous-fated cells. However, the maintenance of arterial-venous fate is susceptible to some degree of plasticity. Thus, in response to signals from the ambient microenvironment and shear stress, there is flow-mediated intercalation of the arteries and veins that ultimately leads to the formation of a functional, closed-loop circulation. In addition, cells in the blood vessels of each organ undergo epigenetic, morphological, and functional adaptive changes that are specific to the proximate function of their cognate organ(s). These adaptive changes result in an interorgan and intraorgan vessel heterogeneity that manifest clinically in a disparate response of different organs to identical risk factors and injury in the same animal. In this review, we focus on the molecular and physiological factors influencing arterial-venous heterogeneity between and within different organ(s). We explore arterial-venous differences in selected organs, as well as their respective endothelial cell architectural organization that results in their inter- and intraorgan heterogeneity.
Conflict of interest statement
Figures
References
-
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. - PubMed
-
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Current Opinion in Cell Biology. 2004;16:580–589. - PubMed
-
- Kullander K, Klein R. Mechanisms and functions of eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. - PubMed
-
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. - PubMed
-
- Wang H, Anderson DJ. Eph Family Transmembrane Ligands Can Mediate Repulsive Guidance of Trunk Neural Crest Migration and Motor Axon Outgrowth. Neuron. 1997;18:383–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

