Two pathways recruit telomerase to Saccharomyces cerevisiae telomeres
- PMID: 18949040
- PMCID: PMC2567097
- DOI: 10.1371/journal.pgen.1000236
Two pathways recruit telomerase to Saccharomyces cerevisiae telomeres
Abstract
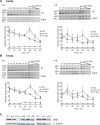
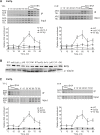
The catalytic subunit of yeast telomerase, Est2p, is a telomere associated throughout most of the cell cycle, while the Est1p subunit binds only in late S/G2 phase, the time of telomerase action. Est2p binding in G1/early S phase requires a specific interaction between telomerase RNA (TLC1) and Ku80p. Here, we show that in four telomerase-deficient strains (cdc13-2, est1A, tlc1-SD, and tlc1-BD), Est2p telomere binding was normal in G1/early S phase but reduced to about 40-50% of wild type levels in late S/G2 phase. Est1p telomere association was low in all four strains. Wild type levels of Est2p telomere binding in late S/G2 phase was Est1p-dependent and required that Est1p be both telomere-bound and associated with a stem-bulge region in TLC1 RNA. In three telomerase-deficient strains in which Est1p is not Est2p-associated (tlc1-SD, tlc1-BD, and est2A), Est1p was present at normal levels but its telomere binding was very low. When the G1/early S phase and the late S/G2 phase telomerase recruitment pathways were both disrupted, neither Est2p nor Est1p was telomere-associated. We conclude that reduced levels of Est2p and low Est1p telomere binding in late S/G2 phase correlated with an est phenotype, while a WT level of Est2p binding in G1 was not sufficient to maintain telomeres. In addition, even though Cdc13p and Est1p interact by two hybrid, biochemical and genetic criteria, this interaction did not occur unless Est1p was Est2p-associated, suggesting that Est1p comes to the telomere only as part of the holoenzyme. Finally, the G1 and late S/G2 phase pathways for telomerase recruitment are distinct and are likely the only ones that bring telomerase to telomeres in wild-type cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



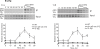
References
-
- Vega L, Mateyak M, Zakian V. Getting to the end: telomerase access in yeast and humans. Nat Rev Mol Cell Biol. 2003;4:948–959. - PubMed
-
- Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. - PubMed
-
- Wellinger RJ, Wolf AJ, Zakian VA. Saccharomyces telomeres acquire single-strand TG1–3 tails late in S phase. Cell. 1993;72:51–60. - PubMed
-
- Wellinger RJ, Ethier K, Labrecque P, Zakian VA. Evidence for a new step in telomere maintenance. Cell. 1996;85:423–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

