Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic
- PMID: 18949058
- PMCID: PMC2571034
- DOI: 10.1172/JCI35437
Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic
Abstract
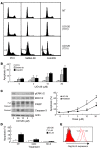
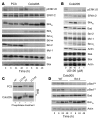
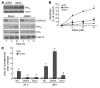
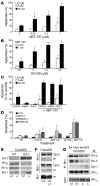
B-RAF is frequently mutated in solid tumors, resulting in activation of the MEK/ERK signaling pathway and ultimately tumor cell growth and survival. MEK inhibition in these cells results in cell cycle arrest and cytostasis. Here, we have shown that MEK inhibition also triggers limited apoptosis of human tumor cell lines with B-RAF mutations and that this effect was dependent on upregulation and dephosphorylation of the proapoptotic, Bcl-2 homology 3-only (BH3-only) Bcl-2 family member Bim. However, upregulation of Bim was insufficient for extensive apoptosis and was countered by overexpression of Bcl-2. To overcome apoptotic resistance, we treated the B-RAF mutant cells both with MEK inhibitors and with the BH3 mimetic ABT-737, resulting in profound synergism and extensive tumor cell death. This treatment was successful because of both efficient antagonism of the prosurvival Bcl-2 family member Mcl-1 by Bim and inhibition of Bcl-2 and Bcl-x(L) by ABT-737. Critically, addition of ABT-737 converted the predominantly cytostatic effect of MEK inhibition to a cytotoxic effect, causing long-term tumor regression in mice xenografted with human tumor cell lines. Thus, the therapeutic efficacy of MEK inhibition requires concurrent unleashing of apoptosis by a BH3 mimetic and represents a potent combination treatment for tumors harboring B-RAF mutations.
Figures


Comment in
-
Anticancer therapy: boosting the bang of Bim.J Clin Invest. 2008 Nov;118(11):3582-4. doi: 10.1172/JCI37553. Epub 2008 Oct 23. J Clin Invest. 2008. PMID: 18949061 Free PMC article.
References
-
- Cohen C., et al. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin. Cancer Res. 2002;8:3728–3733. - PubMed
-
- Brose M.S., et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. - PubMed
-
- Cohen Y., et al. BRAF mutation in papillary thyroid carcinoma. J. Natl. Cancer Inst. 2003;95:625–627. - PubMed
-
- Rajagopalan H., et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

