Signal transduction of fertilization in frog eggs and anti-apoptotic mechanism in human cancer cells: common and specific functions of membrane microdomains
- PMID: 18949075
- PMCID: PMC2570554
- DOI: 10.2174/1874091X00802010049
Signal transduction of fertilization in frog eggs and anti-apoptotic mechanism in human cancer cells: common and specific functions of membrane microdomains
Abstract
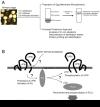
Membrane microdomains or lipid/membrane rafts are distinct areas on the plasma membranes, where a specific subset of lipids (e.g. cholesterol, sphingolipids) and proteins (e.g. glycosylphosphatidylinositol-anchored proteins, growth factor receptor/kinases) are getting together and functioning for several aspects of cellular functions. Our recent investigation has revealed that fertilization of African clawed frog, Xenopus laevis, requires cholesterol-dependent nature of egg membrane microdomains. Moreover, fertilization of Xenopus eggs involves proteolytic cleavage of the extracellular part and subsequent phosphorylation of a cytoplasmic tyrosine residue of uroplakin III, an egg membrane microdomain-associated protein. Protease activity toward uroplakin III seems to be derived from fertilizing sperm, while phosphorylation of uroplakin III seems to be catalyzed by the egg tyrosine kinase Src, whose activation is required for cytoplasmic rearrangement of fertilized eggs; so-called 'egg activation'. Therefore, it is assumed that uroplakin III serves an integral part of signal transduction in fertilization of Xenopus. Our more recent study on human cancer cells has revealed that a similar but distinct scheme of signal transduction operates in anti-apoptotic growth of cells. Namely, in human bladder carcinoma cells, cooperation of uroplakin III and Src, both of which localize to the membrane microdomains, allows cells to escape from apoptotic cell death and proliferate under culture conditions deprived of serum. In this review, I briefly introduce about biology of fertilization and cancer, and then present and discuss our experimental data on general importance and specific features of membrane microdomains in Xenopus fertilization and anti-apoptosis in human bladder carcinoma cells.
Figures



Similar articles
-
The egg membrane microdomain-associated uroplakin III-Src system becomes functional during oocyte maturation and is required for bidirectional gamete signaling at fertilization in Xenopus laevis.Development. 2014 Apr;141(8):1705-14. doi: 10.1242/dev.105510. Development. 2014. PMID: 24715460
-
Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization.J Biol Chem. 2005 Apr 15;280(15):15029-37. doi: 10.1074/jbc.M410538200. Epub 2005 Feb 7. J Biol Chem. 2005. PMID: 15699050
-
Uroplakin III, a novel Src substrate in Xenopus egg rafts, is a target for sperm protease essential for fertilization.Dev Biol. 2005 Oct 15;286(2):483-92. doi: 10.1016/j.ydbio.2005.08.020. Epub 2005 Sep 15. Dev Biol. 2005. PMID: 16168405
-
Gamete membrane microdomains and their associated molecules in fertilization signaling.Mol Reprod Dev. 2011 Oct-Nov;78(10-11):814-30. doi: 10.1002/mrd.21336. Epub 2011 Jun 17. Mol Reprod Dev. 2011. PMID: 21688335 Review.
-
Toward the understanding of biology of oocyte life cycle in Xenopus Laevis: No oocytes left behind.Reprod Med Biol. 2020 Jan 20;19(2):114-119. doi: 10.1002/rmb2.12314. eCollection 2020 Apr. Reprod Med Biol. 2020. PMID: 32273815 Free PMC article. Review.
Cited by
-
Protein phosphatase 2A (PP2A) regulates low density lipoprotein uptake through regulating sterol response element-binding protein-2 (SREBP-2) DNA binding.J Biol Chem. 2014 Jun 13;289(24):17268-79. doi: 10.1074/jbc.M114.570390. Epub 2014 Apr 26. J Biol Chem. 2014. PMID: 24770487 Free PMC article.
-
Lipid rafts: keys to sperm maturation, fertilization, and early embryogenesis.J Lipids. 2011;2011:264706. doi: 10.1155/2011/264706. Epub 2011 Jan 12. J Lipids. 2011. PMID: 21490798 Free PMC article.
-
Uroplakins play conserved roles in egg fertilization and acquired additional urothelial functions during mammalian divergence.Mol Biol Cell. 2018 Dec 15;29(26):3128-3143. doi: 10.1091/mbc.E18-08-0496. Epub 2018 Oct 10. Mol Biol Cell. 2018. PMID: 30303751 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
