Neurocomputational models of basal ganglia function in learning, memory and choice
- PMID: 18950662
- PMCID: PMC2762323
- DOI: 10.1016/j.bbr.2008.09.029
Neurocomputational models of basal ganglia function in learning, memory and choice
Abstract
The basal ganglia (BG) are critical for the coordination of several motor, cognitive, and emotional functions and become dysfunctional in several pathological states ranging from Parkinson's disease to Schizophrenia. Here we review principles developed within a neurocomputational framework of BG and related circuitry which provide insights into their functional roles in behavior. We focus on two classes of models: those that incorporate aspects of biological realism and constrained by functional principles, and more abstract mathematical models focusing on the higher level computational goals of the BG. While the former are arguably more "realistic", the latter have a complementary advantage in being able to describe functional principles of how the system works in a relatively simple set of equations, but are less suited to making specific hypotheses about the roles of specific nuclei and neurophysiological processes. We review the basic architecture and assumptions of these models, their relevance to our understanding of the neurobiological and cognitive functions of the BG, and provide an update on the potential roles of biological details not explicitly incorporated in existing models. Empirical studies ranging from those in transgenic mice to dopaminergic manipulation, deep brain stimulation, and genetics in humans largely support model predictions and provide the basis for further refinement. Finally, we discuss possible future directions and possible ways to integrate different types of models.
Figures

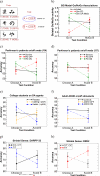
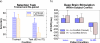
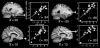
References
-
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levet AI, Forssberg H, Greengrad P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nature Neuroscience. 2000;3:226–230. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. - PubMed
-
- Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. Journal of Neurophysiology. 2004;92(2):1144–1152. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

