Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome
- PMID: 18951087
- PMCID: PMC2614368
- DOI: 10.1016/j.molcel.2008.10.001
Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome
Abstract
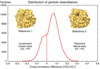
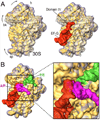
A crucial step in translation is the translocation of tRNAs through the ribosome. In the transition from one canonical site to the other, the tRNAs acquire intermediate configurations, so-called hybrid states. At this stage, the small subunit is rotated with respect to the large subunit, and the anticodon stem loops reside in the A and P sites of the small subunit, while the acceptor ends interact with the P and E sites of the large subunit. In this work, by means of cryo-EM and particle classification procedures, we visualize the hybrid state of both A/P and P/E tRNAs in an authentic factor-free ribosome complex during translocation. In addition, we show how the repositioning of the tRNAs goes hand in hand with the change in the interplay between S13, L1 stalk, L5, H68, H69, and H38 that is caused by the ratcheting of the small subunit.
Figures


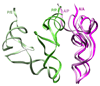
References
-
- Abel KM, Yoder MD, Hilgenfeld R, Jurnak F. An α to β conformational switch in EF-Tu. Structure. 1996;4:1153–1159. - PubMed
-
- Agrawal RK, Penczek P, Grassucci RA, Burkhardt N, Nierhaus KH, Frank J. Effect of buffer conditions on the position of tRNA on the 70S ribosome as visualized by cryoelectron microscopy. J. Biol. Chem. 1999;274:8723–8729. - PubMed
-
- Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell. 2005;121:703–712. - PubMed
-
- Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. tRNA selection and kinetic proofreading in translation. Nat. Struct. Mol. Biol. 2004;11:1008–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

