Azithromycin or montelukast as inhaled corticosteroid-sparing agents in moderate-to-severe childhood asthma study
- PMID: 18951618
- PMCID: PMC2737448
- DOI: 10.1016/j.jaci.2008.09.028
Azithromycin or montelukast as inhaled corticosteroid-sparing agents in moderate-to-severe childhood asthma study
Abstract
Background: Clinical trials in children with moderate-to-severe persistent asthma are limited.
Objective: We sought to determine whether azithromycin or montelukast are inhaled corticosteroid sparing.
Methods: The budesonide dose (with salmeterol [50 microg] twice daily) necessary to achieve control was determined in children 6 to 17 years of age with moderate-to-severe persistent asthma. After a budesonide-stable period of 6 weeks, children were randomized in a double-masked, parallel, multicenter study to receive once-nightly azithromycin, montelukast, or matching placebos plus the established controlling dose of budesonide (minimum, 400 microg twice daily) and salmeterol twice daily. Primary outcome was time from randomization to inadequate asthma control after sequential budesonide dose reduction.
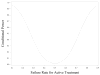
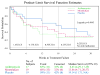
Results: Of 292 children screened, only 55 were randomized. Inadequate adherence to study medication (n = 80) and improved asthma control under close medical supervision (n = 49) were the major reasons for randomization failure. A futility analysis was requested by the Data Safety Monitoring Board. In data available for analyses, no differences were noted for either treatment compared with placebo in time to inadequate control status (median: azithromycin, 8.4 weeks [95% confidence limit, 4.3-17.3]; montelukast, 13.9 weeks [95% confidence limit, 4.7-20.6]; placebo, 19.1 weeks [95% confidence limit, 11.7-infinity]), with no difference between the groups (log-rank test, P = .49). The futility analysis indicated that even if the planned sample size was reached, the results of this negative study were unlikely to be different, and the trial was prematurely terminated.
Conclusion: Based on these results, neither azithromycin nor montelukast is likely to be an effective inhaled corticosteroid-sparing alternative in children with moderate-to-severe persistent asthma.
Figures

References
-
- Moore W, Peters S. Severe asthma: An overview. J Allergy Clin Immunol. 2006;117:487–494. - PubMed
-
- Ball B, Hill M, Brenner M, Sanks R, Szefler S. Effect of low-dose troleandomycin on glucocorticoid kinetics and airway hyperresponsiveness in severely asthmatic children. Annals of Allergy. 1990;65:37–45. - PubMed
-
- Kamada A, Hill M, Iklé D, Brenner A, Szefler S. Efficacy and safety of troleandomycin therapy in severe, steroid-requiring asthmatic children. J Allergy Clin Immunol. 1993;91:873–882. - PubMed
-
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma--Update on selected topics 2002. National Institutes of Health. National Heart, Lung, and Blood Institute; Bethesda, MD.: 2002. National Asthma Education and Prevention Program Expert Panel Report.
-
- National Asthma Education and Prevention Program Expert Panel Report. Expert Panel Report 3:Guidelines for the Diagnosis and Management of Asthma. Department of Health and Human Services; Bethesda, MD: 2007.

