Role of unusual O-glycans in intercellular signaling
- PMID: 18952191
- PMCID: PMC2714165
- DOI: 10.1016/j.biocel.2008.10.001
Role of unusual O-glycans in intercellular signaling
Abstract
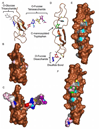
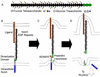
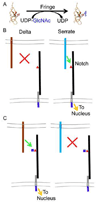
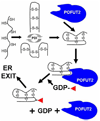
In the last two decades, our knowledge of the role of glycans in development and signal transduction has expanded enormously. While most work has focused on the importance of N-linked or mucin-type O-linked glycosylation, recent work has highlighted the importance of several more unusual forms of glycosylation that are the focus of this review. In particular, the ability of O-fucose glycans on the epidermal growth factor-like (EGF) repeats of Notch to modulate signaling places glycosylation alongside phosphorylation as a means to modulate protein-protein interactions and their resultant downstream signals. The recent discovery that O-glucose modification of Notch EGF repeats is also required for Notch function has further expanded the range of glycosylation events capable of modulating Notch signaling. The prominent role of Notch during development and in later cell-fate decisions underscores the importance of these modifications in human biology. The role of glycans in intercellular signaling events is only beginning to be understood and appears ready to expand into new areas with the discovery that thrombospondin type 1 repeats are also modified with O-fucose glycans. Finally, a rare form of glycosylation called C-mannosylation modifies tryptophans in some signaling competent molecules and may be a further layer of complexity in the field. We will review each of these areas focusing on the glycan structures produced, the consequence of their presence, and the enzymes responsible.
Figures
References
-
- Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Develop. Dynamics. 2000;218:280–299. - PubMed
-
- Andrade RP, Palmeirim I, Bajanca F. Molecular clocks underlying vertebrate embryo segmentation: A 10-year-old hairy-go-round. Birth Defects Res C Embryo Today. 2007;81:65–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

