Antimalarial activity enhancement in hydroxymethylcarbonyl (HMC) isostere-based dipeptidomimetics targeting malarial aspartic protease plasmepsin
- PMID: 18952439
- PMCID: PMC4447328
- DOI: 10.1016/j.bmc.2008.10.011
Antimalarial activity enhancement in hydroxymethylcarbonyl (HMC) isostere-based dipeptidomimetics targeting malarial aspartic protease plasmepsin
Erratum in
- Bioorg Med Chem. 2009 Feb 15;17(4):1772
Abstract
Plasmepsin (Plm) is a potential target for new antimalarial drugs, but most reported Plm inhibitors have relatively low antimalarial activities. We synthesized a series of dipeptide-type HIV protease inhibitors, which contain an allophenylnorstatine-dimethylthioproline scaffold to exhibit potent inhibitory activities against Plm II. Their activities against Plasmodium falciparum in the infected erythrocyte assay were largely different from those against the target enzyme. To improve the antimalarial activity of peptidomimetic Plm inhibitors, we attached substituents on a structure of the highly potent Plm inhibitor KNI-10006. Among the derivatives, we identified alkylamino compounds such as 44 (KNI-10283) and 47 (KNI-10538) with more than 15-fold enhanced antimalarial activity, to the sub-micromolar level, maintaining their potent Plm II inhibitory activity and low cytotoxicity. These results suggest that auxiliary substituents on a specific basic group contribute to deliver the inhibitors to the target Plm.
Figures


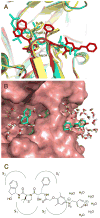

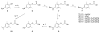


References
-
- World Malaria Report 2005. WHO and UNICEF; 2005.
-
- Olliaro PL, Bloland PB. In: Antimalarial Chemotherapy. Rosenthal PJ, editor. Humana Press; Totawa, NJ: 2001. pp. 65–83.
-
- Ridley RG. Nature. 2002;415:686. - PubMed
-
- Benerjee R, Goldberg DE. In: Antimalarial Chemotherapy. Rosenthal PJ, editor. Humana Press; Totawa, NJ: 2001. pp. 43–63.
-
- Cooms GH, Goldberg DE, Klemba M, Berry C, Kay J, Mottram JC. Trends Parasitol. 2001;17:532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

