Tyrosine-sulfate isosteres of CCR5 N-terminus as tools for studying HIV-1 entry
- PMID: 18952441
- PMCID: PMC2650494
- DOI: 10.1016/j.bmc.2008.10.005
Tyrosine-sulfate isosteres of CCR5 N-terminus as tools for studying HIV-1 entry
Abstract
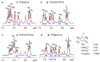
The HIV-1 co-receptor CCR5 possesses sulfo-tyrosine (TYS) residues at its N-terminus (Nt) that are required for binding HIV-1 gp120 and mediating viral entry. By using a 14-residue fragment of CCR5 Nt containing two TYS residues, we recently showed that CCR5 Nt binds gp120 through a conserved region specific for TYS moieties and suggested that this site may represent a target for inhibitors and probes of HIV-1 entry. As peptides containing sulfo-tyrosines are difficult to synthesize and handle due to limited stability of the sulfo-ester moiety, we have now incorporated TYS isosteres into CCR5 Nt analogs and assessed their binding to a complex of gp120-CD4 using saturation transfer difference (STD) NMR and surface plasmon resonance (SPR). STD enhancements for CCR5 Nt peptides containing tyrosine sulfonate (TYSN) in complex with gp120-CD4 were very similar to those observed for sulfated CCR5 Nt peptides indicating comparable modes of binding. STD enhancements for phosphotyrosine-containing CCR5 Nt analogs were greatly diminished consistent with earlier findings showing sulfo-tyrosine to be essential for CCR5 Nt binding to gp120. Tyrosine sulfonate-containing CCR5 peptides exhibited reduced water solubility, limiting their use in assay and probe development. To improve solubility, we designed, synthesized, and incorporated in CCR5 Nt peptide analogs an orthogonally functionalized azido tris(ethylenoxy) l-alanine (l-ate-Ala) residue. Through NMR and SPR experiments, we show a 19-residue TYSN-containing peptide to be a functional, hydrolytically stable CCR5 Nt isostere that was in turn used to develop both SPR-based and ELISA assays to screen for inhibitors of CCR5 binding to gp120-CD4.
Figures
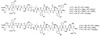




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

