Cyclooxygenases: structural and functional insights
- PMID: 18952571
- PMCID: PMC2674713
- DOI: 10.1194/jlr.R800042-JLR200
Cyclooxygenases: structural and functional insights
Abstract
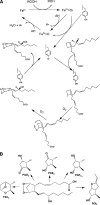
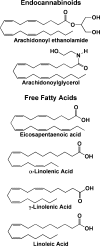
Cyclooxygenase (COX; prostaglandin G/H synthase, EC 1.14.99.1) catalyzes the first two steps in the biosynthesis of prostaglandins (PGs). The two COX isoforms COX-1 and COX-2 are the targets of the widely used nonsteroidal anti-inflammatory drugs, indicating a role for these enzymes in pain, fever, inflammation, and tumorigenesis. The ubiquitous constitutive expression of COX-1 and inducible expression of COX-2 have led to the widely held belief that COX-1 produces homeostatic PGs, while PGs produced by COX-2 are primarily pathophysiological. However, recent discoveries call this paradigm into question and reveal as yet underappreciated functions for both enzymes. This review focuses on some of these new insights.
Figures
References
-
- Smith W. L., D. L. DeWitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69 145–182. - PubMed
-
- Rouzer C. A., and L. J. Marnett. 2003. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 103 2239–2304. - PubMed
-
- van der Donk W. A., A. L. Tsai, and R. J. Kulmacz. 2002. The cyclooxygenase reaction mechanism. Biochemistry. 41 15451–15458. - PubMed
-
- Samuelsson B., M. Goldyne, E. Granstrom, M. Hamberg, S. Hammarstrom, and C. Malmsten. 1978. Prostaglandins and thromboxanes. Annu. Rev. Biochem. 47 997–1029. - PubMed
-
- Hata A. N., and R. M. Breyer. 2004. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103 147–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

