Role of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic target
- PMID: 18952846
- PMCID: PMC2582249
- DOI: 10.1073/pnas.0804054105
Role of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic target
Abstract
Cicatricial contraction of preretinal fibrous membrane is a cause of severe vision loss in proliferative vitreoretinal diseases such as proliferative diabetic retinopathy (PDR) and proliferative vitreoretinopathy (PVR). TGF-beta is overexpressed in the vitreous of patients with proliferative vitreoretinal diseases and is also detectable in the contractile membranes. Therefore, TGF-beta is presumed to contribute to the cicatricial contraction of the membranes, however, the underlying mechanisms and TGF-beta's importance among various other factors remain to be elucidated. Vitreous samples from PDR or PVR patients caused significantly larger contraction of hyalocyte-containing collagen gels, compared with nonproliferative controls. The contractile effect was strongly correlated with the vitreal concentration of activated TGF-beta2 (r = 0.82, P < 0.0001). PDR or PVR vitreous promoted expression of alpha-smooth muscle actin (alpha-SMA) and phosphorylation of myosin light chain (MLC), a downstream mediator of Rho-kinase (ROCK), both of which were dramatically but incompletely suppressed by TGF-beta blockade. In contrast, fasudil, a potent and selective ROCK inhibitor, almost completely blocked the vitreous-induced MLC phosphorylation and collagen gel contraction. Fasudil disrupted alpha-SMA organization, but it did not affect its vitreal expression. In vivo, fasudil significantly inhibited the progression of experimental PVR in rabbit eyes without affecting the viability of retinal cells by electroretinographic and histological analyses. These results elucidate the critical role of TGF-beta in mediating cicatricial contraction in proliferative vitreoretinal diseases. ROCK, a key downstream mediator of TGF-beta and other factors might become a unique therapeutic target in the treatment of proliferative vitreoretinal diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

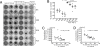
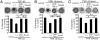
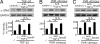
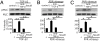

References
-
- Fong DS, et al. American Diabetes Association: Diabetic retinopathy. Diabetes Care. 2003;26:S99–S102. - PubMed
-
- Pastor JC, de la Rua ER, Martin F. Proliferative vitreoretinopathy: Risk factors and pathology. Prog Retin Eye Res. 2002;21:127–144. - PubMed
-
- Kampik A, Kenyon KR, Michels RG, Green WR, de la Cruz ZC. Epiretinal and vitreous membranes: Comparative study of 56 cases. Arch Ophthalmol. 1981;99:1445–1454. - PubMed
-
- Jerdan JA, et al. Proliferative vitreoretinopathy membranes: An immunohistochemical study. Ophthalmology. 1989;96:801–810. - PubMed
-
- Salu P, Claeskens W, De Wilde A, Hijmans W, Wisse E. Light and electron microscopic studies of the rat hyalocyte after perfusion fixation. Ophthalmic Res. 1985;17:125–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

