Beta-arrestin 2 is required for lysophosphatidic acid-induced NF-kappaB activation
- PMID: 18952848
- PMCID: PMC2579382
- DOI: 10.1073/pnas.0802701105
Beta-arrestin 2 is required for lysophosphatidic acid-induced NF-kappaB activation
Abstract
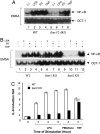
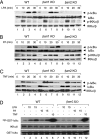
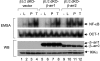
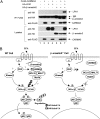
Lysophosphatidic acid (LPA) is a bioactive phospholipid and binds to its receptors, a family of G protein-coupled receptors (GPCR), which initiates multiple signaling cascades and leads to activation of several transcription factors, including NF-kappaB. Although LPA-induced signaling pathways have been intensively investigated, the molecular mechanism by which LPA activates NF-kappaB is not fully defined. In this work, we found that beta-arrestin 2, but not beta-arrestin 1, is required for LPA-induced NF-kappaB activation and interlukin-6 expression. Mechanistically, we found that beta-arrestin 2 associated with CARMA3, a scaffold protein that plays an essential role in GPCR-induced NF-kappaB activation, suggesting that beta-arrestin 2 may recruit CARMA3 to LPA receptors. Although beta-arrestin 2 deficiency did not affect LPA-induced IKKalpha/beta phosphorylation, it impaired LPA-induced IKK kinase activity, which is consistent with our previous findings that CARMA3 is required for IKKalpha/beta activation but not for the inducible phosphorylation of IKKalpha/beta. Together, our results provide the genetic evidence that beta-arrestin 2 serves as a positive regulator in NF-kappaB signaling pathway by connecting CARMA3 to GPCRs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. - PubMed
-
- Ye RD. Regulation of nuclear factor κB activation by G protein-coupled receptors. J Leukocyte Biol. 2001;70:839–848. - PubMed
-
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. - PubMed
-
- Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. - PubMed
-
- Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

