Control of lipid accumulation in the yeast Yarrowia lipolytica
- PMID: 18952867
- PMCID: PMC2607157
- DOI: 10.1128/AEM.01412-08
Control of lipid accumulation in the yeast Yarrowia lipolytica
Abstract
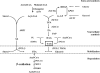
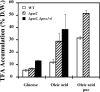
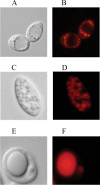
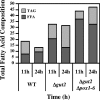
A genomic comparison of Yarrowia lipolytica and Saccharomyces cerevisiae indicates that the metabolism of Y. lipolytica is oriented toward the glycerol pathway. To redirect carbon flux toward lipid synthesis, the GUT2 gene, which codes for the glycerol-3-phosphate dehydrogenase isomer, was deleted in Y. lipolytica in this study. This Delta gut2 mutant strain demonstrated a threefold increase in lipid accumulation compared to the wild-type strain. However, mobilization of lipid reserves occurred after the exit from the exponential phase due to beta-oxidation. Y. lipolytica contains six acyl-coenzyme A oxidases (Aox), encoded by the POX1 to POX6 genes, that catalyze the limiting step of peroxisomal beta-oxidation. Additional deletion of the POX1 to POX6 genes in the Delta gut2 strain led to a fourfold increase in lipid content. The lipid composition of all of the strains tested demonstrated high proportions of FFA. The size and number of the lipid bodies in these strains were shown to be dependent on the lipid composition and accumulation ratio.
Figures



References
-
- Aggelis, G., and J. Sourdis. 1997. Prediction of lipid accumulation-degradation in oleaginous micro-organisms growing on vegetable oils. Antonie van Leeuwenhoek 72:159-165. - PubMed
-
- Athenstaedt, K., P. Jolivet, C. Boulard, M. Zivy, L. Negroni, J. M. Nicaud, and T. Chardot. 2006. Lipid particle composition of the yeast Yarrowia lipolytica depends on the carbon source. Proteomics 6:1450-1459. - PubMed
-
- Barth, G., J. M. Beckerich, A. Dominguez, S. Kerscher, D. Ogrydziak, V. Titorenko, and C. Gaillardin. 2003. Functional genetics of Yarrowia lipolytica, p. 227-271. In J. H. de Winde (ed.), Functional genetics of industrial yeasts, vol. 1. Springer-Verlag, Berlin, Germany.
-
- Barth, G., and C. Gaillardin. 1996. Yarrowia lipolytica, p. 313-388. In K. Wolf, K. D. Breunig, and G. Barth (ed.), Nonconventional yeasts in biotechnology, vol. 1. Springer-Verlag, Berlin, Germany.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

