Glutathione S-transferase M1 and T1 polymorphisms may predict adverse effects after therapy in children with medulloblastoma
- PMID: 18952980
- PMCID: PMC2718973
- DOI: 10.1215/15228517-2008-089
Glutathione S-transferase M1 and T1 polymorphisms may predict adverse effects after therapy in children with medulloblastoma
Abstract
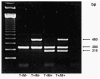
Glutathione S-transferases (GSTs) are polymorphic enzymes that catalyze the glutathione conjugation of alkylating agents, platinum compounds, and free radicals formed by radiation used to treat medulloblastoma. We hypothesized that GST polymorphisms may be responsible, in part, for individual differences in toxicity and responses in pediatric medulloblastoma. We investigated the relationship between GSTM1 and GSTT1 polymorphisms and survival and toxicity in 42 children with medulloblastoma diagnosed and treated at the Texas Children's Cancer Center. We conducted Kaplan-Meier analyses to determine if the GST polymorphisms were related to progression-free survival (PFS) and performed logistic regression to explore associations between GST polymorphisms and occurrence of grade 3 or greater (> or =Gr 3) myelosuppression, ototoxicity, nephrotoxicity, neurotoxicity, and intellectual impairment. Patients with at least one null genotype had a 4.3 (95% confidence interval, 1.1-16.8), 3.7 (1-13.6), and 6.4 (1.2-34) times increased risk for any > or =Gr 3 toxicity, any > or =Gr 3 toxicity excluding peripheral neuropathy, and any > or =Gr 3 toxicity requiring omission or cessation of chemotherapy, respectively. Compared with all others, patients with at least one null genotype had, on average, 27.2 (p x= 0.0002), 29 (p = 0.0004), and 21.7 (p = 0.002) lower full-scale, performance, and verbal intelligence quotient (IQ) scores, respectively. GSTM1 and GSTT1 polymorphisms may predict adverse events, including cognitive impairment after therapy, in patients with medulloblastoma. A larger study to validate these findings is under way.
Figures

References
-
- Blaney SM, Kun L, Hunter J, et al. Tumors of the central nervous system. In: Pizzo PA, Poplack D, editors. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams and Wilkins; 2006. pp. 786–864.
-
- Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurgery. 1996;38(2):265–271. - PubMed
-
- Evans AE, Jenkin RD, Sposto R, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg. 1990;72(4):572–582. - PubMed
-
- Packer RJ, Sutton LN, Elterman R, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg. 1994;81(5):690–698. - PubMed
-
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

