REV1 restrains DNA polymerase zeta to ensure frame fidelity during translesion synthesis of UV photoproducts in vivo
- PMID: 18953031
- PMCID: PMC2588525
- DOI: 10.1093/nar/gkn651
REV1 restrains DNA polymerase zeta to ensure frame fidelity during translesion synthesis of UV photoproducts in vivo
Abstract
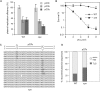
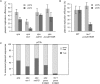
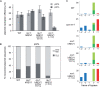
Exposure to ultraviolet light induces a number of forms of damage in DNA, of which (6-4) photoproducts present the most formidable challenge to DNA replication. No single DNA polymerase has been shown to bypass these lesions efficiently in vitro suggesting that the coordinate use of a number of different enzymes is required in vivo. To further understand the mechanisms and control of lesion bypass in vivo, we have devised a plasmid-based system to study the replication of site-specific T-T(6-4) photoproducts in chicken DT40 cells. We show that DNA polymerase zeta is absolutely required for translesion synthesis (TLS) of this lesion, while loss of DNA polymerase eta has no detectable effect. We also show that either the polymerase-binding domain of REV1 or ubiquitinated PCNA is required for the recruitment of Polzeta as the catalytic TLS polymerase. Finally, we demonstrate a previously unappreciated role for REV1 in ensuring bypass synthesis remains in frame with the template. Our data therefore suggest that REV1 not only helps to coordinate the delivery of DNA polymerase zeta to a stalled primer terminus but also restrains its activity to ensure that nucleotides are incorporated in register with the template strand.
Figures




References
-
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 2005;74:317–353. - PubMed
-
- Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. - PubMed
-
- Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase η with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell. 2004;14:491–500. - PubMed
-
- Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science. 2005;310:1821–1824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

