Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions
- PMID: 18953331
- PMCID: PMC2692841
- DOI: 10.1038/nature07409
Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions
Retraction in
-
Retraction: Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions.Nature. 2014 Jul 17;511(7509):370. doi: 10.1038/nature13549. Nature. 2014. PMID: 25030176 Free PMC article. No abstract available.
Abstract
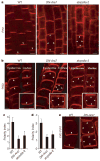
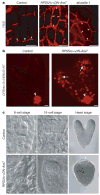
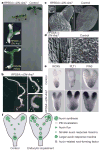
Dynamically polarized membrane proteins define different cell boundaries and have an important role in intercellular communication-a vital feature of multicellular development. Efflux carriers for the signalling molecule auxin from the PIN family are landmarks of cell polarity in plants and have a crucial involvement in auxin distribution-dependent development including embryo patterning, organogenesis and tropisms. Polar PIN localization determines the direction of intercellular auxin flow, yet the mechanisms generating PIN polarity remain unclear. Here we identify an endocytosis-dependent mechanism of PIN polarity generation and analyse its developmental implications. Real-time PIN tracking showed that after synthesis, PINs are initially delivered to the plasma membrane in a non-polar manner and their polarity is established by subsequent endocytic recycling. Interference with PIN endocytosis either by auxin or by manipulation of the Arabidopsis Rab5 GTPase pathway prevents PIN polarization. Failure of PIN polarization transiently alters asymmetric auxin distribution during embryogenesis and increases the local auxin response in apical embryo regions. This results in ectopic expression of auxin pathway-associated root-forming master regulators in embryonic leaves and promotes homeotic transformation of leaves to roots. Our results indicate a two-step mechanism for the generation of PIN polar localization and the essential role of endocytosis in this process. It also highlights the link between endocytosis-dependent polarity of individual cells and auxin distribution-dependent cell fate establishment for multicellular patterning.
Figures

References
-
- Petrasek J, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. - PubMed
-
- Galweiler L, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. - PubMed
-
- Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. - PubMed
-
- Benkova E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. - PubMed
-
- Reinhardt D, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

