Unexpected power-law stress relaxation of entangled ring polymers
- PMID: 18953345
- PMCID: PMC4819970
- DOI: 10.1038/nmat2292
Unexpected power-law stress relaxation of entangled ring polymers
Abstract
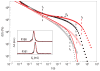
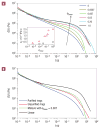
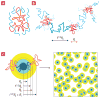
After many years of intense research, most aspects of the motion of entangled polymers have been understood. Long linear and branched polymers have a characteristic entanglement plateau and their stress relaxes by chain reptation or branch retraction, respectively. In both mechanisms, the presence of chain ends is essential. But how do entangled polymers without ends relax their stress? Using properly purified high-molar-mass ring polymers, we demonstrate that these materials exhibit self-similar dynamics, yielding a power-law stress relaxation. However, trace amounts of linear chains at a concentration almost two decades below their overlap cause an enhanced mechanical response. An entanglement plateau is recovered at higher concentrations of linear chains. These results constitute an important step towards solving an outstanding problem of polymer science and are useful for manipulating properties of materials ranging from DNA to polycarbonate. They also provide possible directions for tuning the rheology of entangled polymers.
Figures
Comment in
-
Polymer dynamics: Floored by the rings.Nat Mater. 2008 Dec;7(12):933-5. doi: 10.1038/nmat2324. Nat Mater. 2008. PMID: 19029927 No abstract available.
References
-
- Rouse PE. A theory of the linear viscoelastic properties of dilute solutions of coiling polymers. J Chem Phys. 1953;21:1272–1280.
-
- Doi M, Edwards SF. The Theory of Polymer Dynamics. Oxford Univ. Press; 1986.
-
- Rubinstein M, Colby RH. Polymer Physics. Oxford Univ. Press; 2003.
-
- De Gennes PG. Reptation of a polymer chain in a presence of fixed obstacles. J Chem Phys. 1971;35:572–579.
-
- Doi M, Edwards SF. Dynamics of concentrated polymer systems. Part 1 Brownian motion in the equilibrium state. J Chem Soc Faraday Trans 2. 1978;74:1789–1801.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

