Wnt5a regulates ventral midbrain morphogenesis and the development of A9-A10 dopaminergic cells in vivo
- PMID: 18953410
- PMCID: PMC2568809
- DOI: 10.1371/journal.pone.0003517
Wnt5a regulates ventral midbrain morphogenesis and the development of A9-A10 dopaminergic cells in vivo
Abstract
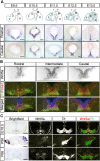
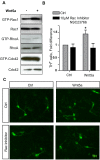
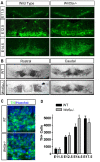
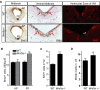
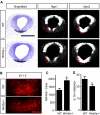
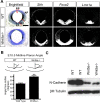
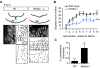
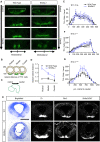
Wnt5a is a morphogen that activates the Wnt/planar cell polarity (PCP) pathway and serves multiple functions during development. PCP signaling controls the orientation of cells within an epithelial plane as well as convergent extension (CE) movements. Wnt5a was previously reported to promote differentiation of A9-10 dopaminergic (DA) precursors in vitro. However, the signaling mechanism in DA cells and the function of Wnt5a during midbrain development in vivo remains unclear. We hereby report that Wnt5a activated the GTPase Rac1 in DA cells and that Rac1 inhibitors blocked the Wnt5a-induced DA neuron differentiation of ventral midbrain (VM) precursor cultures, linking Wnt5a-induced differentiation with a known effector of Wnt/PCP signaling. In vivo, Wnt5a was expressed throughout the VM at embryonic day (E)9.5, and was restricted to the VM floor and basal plate by E11.5-E13.5. Analysis of Wnt5a-/- mice revealed a transient increase in progenitor proliferation at E11.5, and a precociously induced NR4A2+ (Nurr1) precursor pool at E12.5. The excess NR4A2+ precursors remained undifferentiated until E14.5, when a transient 25% increase in DA neurons was detected. Wnt5a-/- mice also displayed a defect in (mid)brain morphogenesis, including an impairment in midbrain elongation and a rounded ventricular cavity. Interestingly, these alterations affected mostly cells in the DA lineage. The ventral Sonic hedgehog-expressing domain was broadened and flattened, a typical CE phenotype, and the domains occupied by Ngn2+ DA progenitors, NR4A2+ DA precursors and TH+ DA neurons were rostrocaudally reduced and laterally expanded. In summary, we hereby describe a Wnt5a regulation of Wnt/PCP signaling in the DA lineage and provide evidence for multiple functions of Wnt5a in the VM in vivo, including the regulation of VM morphogenesis, DA progenitor cell division, and differentiation of NR4A2+ DA precursors.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

