The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP
- PMID: 18953429
- PMCID: PMC2570596
- DOI: 10.1593/neo.08642
The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP
Abstract
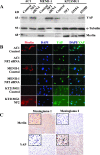
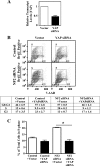
Neurofibromatosis type 2 (NF2) is an autosomal dominant disorder characterized by the occurrence of schwannomas and meningiomas. Several studies have examined the ability of the NF2 gene product, merlin, to function as a tumor suppressor in diverse cell types; however, little is known about merlin growth regulation in meningiomas. In Drosophila, merlin controls cell proliferation and apoptosis by signaling through the Hippo pathway to inhibit the function of the transcriptional coactivator Yorkie. The Hippo pathway is conserved in mammals. On the basis of these observations, we developed human meningioma cell lines matched for merlin expression to evaluate merlin growth regulation and investigate the relationship between NF2 status and Yes-associated protein (YAP), the mammalian homolog of Yorkie. NF2 loss in meningioma cells was associated with loss of contact-dependent growth inhibition, enhanced anchorage-independent growth and increased cell proliferation due to increased S-phase entry. In addition, merlin loss in both meningioma cell lines and primary tumors resulted in increased YAP expression and nuclear localization. Finally, siRNA-mediated reduction of YAP in NF2-deficient meningioma cells rescued the effects of merlin loss on cell proliferation and S-phase entry. Collectively, these results represent the first demonstration that merlin regulates cell growth in human cancer cells by suppressing YAP.
Figures





References
-
- Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–351. - PubMed
-
- Ruttledge MH, Sarrazin J, Rangaratnam S, Phelan CM, Twist E, Merel P, Delattre O, Thomas G, Nordenskjold M, Collins VP, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994;6:180–184. - PubMed
-
- Gautreau A, Louvard D, Arpin M. ERM proteins and NF2 tumor suppressor: the yin and yang of cortical actin organization and cell growth signaling. Curr Opin Cell Biol. 2002;14:104–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
