Detection and characterization of chicken anemia virus from commercial broiler breeder chickens
- PMID: 18954433
- PMCID: PMC2605446
- DOI: 10.1186/1743-422X-5-128
Detection and characterization of chicken anemia virus from commercial broiler breeder chickens
Abstract
Background: Chicken anemia virus (CAV) is the causative agent of chicken infectious anemia (CIA). Study on the type of CAV isolates present and their genetic diversity, transmission to their progeny and level of protection afforded in the breeder farms is lacking in Malaysia. Hence, the present study was aimed to detect CAV from commercial broiler breeder farms and characterize CAV positive samples based on sequence and phylogenetic analysis of partial VP1 gene.
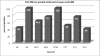
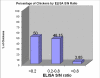
Results: A total of 12 CAV isolates from different commercial broiler breeder farms were isolated and characterized. Detection of CAV positive embryos by the PCR assay in the range of 40 to 100% for different farms indicated high level of occurrence of vertical transmission of viral DNA to the progeny. CAV antigen was detected in the thymus and in the bone marrow but not in spleen, liver, duodenum, ovary and oviduct by indirect immunoperoxidase staining. The 12 CAV isolates were characterized based on partial sequences of VP1 gene. Six isolates (MF1A, MF3C, M3B5, NF4A, P12B and P24A) were found to have maximum homology with previously characterized Malaysian isolate SMSC-1, four isolates (M1B1, NF3A, PYT4 and PPW4) with isolate BL-5 and the remaining two (NF1D and NF2C) have maximum homology both with isolates 3-1 and BL-5. Meanwhile, seven of the isolates with amino acid profile of 75-I, 97-L, 139-Q and 144-Q were clustered together in cluster I together with other isolates from different geographical places. The remaining five isolates with amino acid profile of 75-V, 97-M, 139-K and 144-E were grouped under cluster II. All the CAV isolates demonstrated omega values (Ka/Ks) of less than one (the values ranging from 0.07 to 0.5) suggesting the occurrence of purifying (negative) selection in all the studied isolates.
Conclusion: The present study showed that CAV is widespread in the studied commercial broiler breeder farms. The result also indicated the occurrence of genetic variability in local CAV isolates that can be divided at least into two groups based on characteristic amino acid substitutions at positions 75, 97, 139 and 144 of the VP1 protein.
Figures



References
-
- Schat KA, Circovirus infections . Chicken infectious anemia. In: Saif YM, Barnes HJ, Fadly AM, Glisson JR, McDougald LR, Swayne DE, editor. Diseases of Poultry. Iowa State University Press, Ames, IA; 2003. pp. 182–202.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

