Understanding breast cancer patients' preference for two types of exercise training during chemotherapy in an unblinded randomized controlled trial
- PMID: 18954442
- PMCID: PMC2582030
- DOI: 10.1186/1479-5868-5-52
Understanding breast cancer patients' preference for two types of exercise training during chemotherapy in an unblinded randomized controlled trial
Abstract
Background: Patient preference for group assignment may affect outcomes in unblinded trials but few studies have attempted to understand such preferences. The purpose of the present study was to examine factors associated with breast cancer patients' preference for two types of exercise training during chemotherapy.
Methods: Breast cancer patients (N = 242) completed a battery of tests including a questionnaire that assessed patient preference and the theory of planned behavior (TPB) prior to being randomized to usual care, resistance exercise training (RET), or aerobic exercise training (AET).
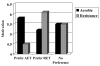
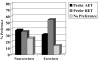
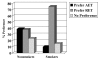
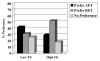
Results: 99 (40.9%) participants preferred RET, 88 (36.4%) preferred AET, and 55 (22.7%) reported no preference. Past exercisers (p = 0.023), smokers (p = 0.004), and aerobically fitter participants (p = 0.005) were more likely to prefer RET. As hypothesized, participants that preferred AET had more favorable TPB beliefs about AET whereas participants that preferred RET had more favorable TPB beliefs about RET. In multivariate modeling, patient preference for RET versus AET was explained (R2 = .46; p < 0.001) by the difference in motivation for RET versus AET (beta = .56; p < 0.001), smoking status (beta = .13; p = 0.007), and aerobic fitness (beta = .12; p = 0.018). Motivational difference between RET versus AET, in turn, was explained (R2 = .48; p < 0.001) by differences in instrumental attitude (beta = .27; p < 0.001), affective attitude (beta = .25; p < 0.001), and perceived behavioral control (beta = .24; p < 0.001).
Conclusion: Breast cancer patients' preference for RET versus AET during chemotherapy was predicted largely by a difference in motivation for each type of exercise which, in turn, was based on differences in their beliefs about the anticipated benefits, enjoyment, and difficulty of performing each type of exercise during chemotherapy. These findings may help explain patient preference effects in unblinded behavioral trials.
Trial registration: ClinicalTrials.gov Identifier NCT00115713.
Figures
References
-
- Moher D, Schulz KF, Altman D. The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel-Group Randomized Trials. Explore (NY) 2005;1:40–45. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

