Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion
- PMID: 18955382
- PMCID: PMC2655417
- DOI: 10.1113/jphysiol.2008.160333
Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion
Abstract
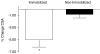
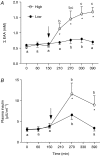
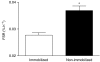
We tested the hypothesis that increasing blood amino acid (AA) availability would counter the physical inactivity-induced reduction in muscle protein synthesis. We determined how 14 days of unilateral knee immobilization affected quadriceps myofibrillar protein synthesis (MPS) in young healthy subjects (10 men, 2 women, 21 +/- 1 years; 80.2 +/- 4.0 kg, mean +/- S.E.M.) in the post-absorptive state and after infusing AA (10% Primene) at low or high doses (43 and 261 mg kg(-1) h(-1)). Muscle cross-sectional area (MRI) and peak isometric torque declined in the immobilized leg (-5.0 +/- 1.2% and -25 +/- 3%, respectively, both P < 0.005), but were unchanged (all P > 0.6) in the non-immobilized leg. Immobilization induced a 27% decline in the rate of post-absorptive MPS (immobilized, 0.027 +/- 0.003: non-immobilized, 0.037 +/- 0.003% h(-1); P < 0.001). Regardless of dose, AA infusion stimulated a greater rise in MPS in the non-immobilized legs; at 4 h MPS was greater by +54 +/- 12% with low dose and +68 +/- 17% with high dose AA infusion (both P < 0.001). There was some evidence of delayed responsiveness of phosphorylation of Akt to high doses of AA and p70S6k at both doses but no marked differences in that of mTOR, GSK3beta or eEF2. Phosphorylation of focal adhesion kinase (Tyr(576/577)) was reduced (P < 0.05) with immobilization. We observed no change in polyubiquitinated protein content after immobilization. We confirm that 14 days of immobilization reduces MPS in the post-absorptive state and this diminution is reduced but not abolished by increased provision of AA, even at high rates. The immobilization-induced decline in post-absorptive MPS with the 'anabolic resistance' to amino acids can account for much of immobilization-induced muscle atrophy.
Figures




References
-
- Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185–2201. - PubMed
-
- Alkner BA, Tesch PA. Efficacy of a gravity-independent resistance exercise device as a countermeasure to muscle atrophy during 29-day bed rest. Acta Physiol Scand. 2004;181:345–357. - PubMed
-
- Anastasi G, Cutroneo G, Santoro G, Arco A, Rizzo G, Trommino C, Bramanti P, Soscia L, Favaloro A. Integrins, muscle agrin and sarcoglycans during muscular inactivity conditions: an immunohistochemical study. Eur J Histochem. 2006;50:327–336. - PubMed
-
- Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–6435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

