Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy
- PMID: 18955671
- PMCID: PMC2777708
- DOI: 10.1161/CIRCULATIONAHA.107.748962
Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy
Abstract
Background: Sudden cardiac death remains a leading cause of mortality despite advances in medical treatment for the prevention of ischemic heart disease and heart failure. Recent studies showed a benefit of implantable cardioverter defibrillator implantation, but appropriate shocks for ventricular tachyarrhythmias were noted only in a minority of patients during 4 to 5 years of follow-up. Accordingly, better risk stratification is needed to optimize patient selection. In this regard, microvolt T-wave alternans (TWA) has emerged as a potentially useful measure of arrhythmia vulnerability, but it has not been evaluated previously in a prospective, randomized trial of implantable cardioverter defibrillator therapy.
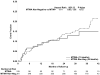
Methods and results: This investigation was a prospective substudy of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) that included 490 patients at 37 clinical sites. TWA tests were classified by blinded readers as positive (37%), negative (22%), or indeterminate (41%) by standard criteria. The composite primary end point was the first occurrence of any of the following events: sudden cardiac death, sustained ventricular tachycardia/fibrillation, or appropriate implantable cardioverter defibrillator discharge. During a median follow-up of 30 months, no significant differences in event rates were found between TWA-positive or -negative patients (hazard ratio 1.24, 95% confidence interval 0.60 to 2.59, P=0.56) or TWA-negative and nonnegative (positive and indeterminate) subjects (hazard ratio 1.28, 95% confidence interval 0.65 to 2.53, P=0.46). Similar results were obtained with the inclusion or exclusion of patients randomized to amiodarone in the analyses.
Conclusions: TWA testing did not predict arrhythmic events or mortality in SCD-HeFT, although a small reduction in events (20% to 25%) among TWA-negative patients cannot be excluded given the sample size of this study. Accordingly, these results suggest that TWA is not useful as an aid in clinical decision making on implantable cardioverter defibrillator therapy among patients with heart failure and left ventricular systolic dysfunction.
Conflict of interest statement
Conflict of Interest Disclosures:
Michael R Gold: Research Grant Cambridge Heart, Consultant: Medtronic, Boston Scientific, St Jude John I Ip: None Otto Costantini: Consultant: Boston Scientific, St Jude Jeanne Poole: Research Grant HAT, Biotronik, Speaker Bureau Boston Scientific, Sorin, Medtronic, Biotronik Steven McNulty: None Daniel B Mark: Research Grant: Medtronic, Consultant Medtronic Kerry L Lee: Research Grant Cambridge Heart, Honoraria Medtronic Gust H Bardy Research Grant NIH, Medtronic, Philips, Laerdal, Ownership Interest Cameron Health, Consultant Philips, Boston Scientific, Institution/Employer Seattle Institute for Cardiac Research
Figures


Comment in
-
T-wave alternans in the sudden cardiac death in heart failure trial population: signal or noise?Circulation. 2008 Nov 11;118(20):2015-8. doi: 10.1161/CIRCULATIONAHA.108.818286. Circulation. 2008. PMID: 19001031 No abstract available.
-
Letter by De Ferrari and Verrier regarding article, "Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy".Circulation. 2009 Jul 21;120(3):e20; author reply e22. doi: 10.1161/CIRCULATIONAHA.108.838128. Circulation. 2009. PMID: 19620521 No abstract available.
-
Letter by Madias regarding article, "Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy".Circulation. 2009 Jul 21;120(3):e21; author reply e22. doi: 10.1161/CIRCULATIONAHA.108.834747. Circulation. 2009. PMID: 19620522 No abstract available.
References
-
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. Multicenter Automatic Defibrillator Implantation Trial Investigators. - PubMed
-
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G, Multicenter Unsustained Tachycardia Trial Investigators A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. - PubMed
-
- The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. - PubMed
-
- Connolly S, Gent M, Roberts R, Dorian P, Roy D, Sheldon R, Mitchell L, Green M, Klein G, O'Brien B. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. - PubMed
-
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

