Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease
- PMID: 18956292
- PMCID: PMC2908270
- DOI: 10.1055/s-0028-1091980
Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease
Abstract
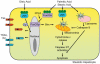
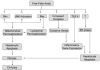
Nonalcoholic fatty liver disease (NAFLD) is characterized by insulin resistance, which results in elevated serum concentration of free fatty acids (FFAs). Circulating FFAs provide the substrate for triacylglycerol formation in the liver, and may also be directly cytotoxic. Hepatocyte apoptosis is a key histologic feature of NAFLD, and correlates with progressive inflammation and fibrosis. The molecular pathways leading to hepatocyte apoptosis are not fully defined; however, recent studies suggest that FFA-induced apoptosis contributes to the pathogenesis of nonalcoholic steatohepatitis. FFAs directly engage the core apoptotic machinery by activating the proapoptotic protein Bax, in a c-jun N-terminal kinase-dependent manner. FFAs also activate the lysosomal pathway of cell death and regulate death receptor gene expression. The role of ER stress and oxidative stress in the pathogenesis of nonalcoholic steatohepatitis has also been described. Understanding the molecular mediators of liver injury should promote development of mechanism-based therapeutic interventions.
Figures
References
-
- Unger RH. Minireview: weapons of lean body mass destruction—the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144(12):5159–5165. - PubMed
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. - PubMed
-
- Gregor MF, Hotamisligil GS. Thematic review series: adipocyte biology. adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48(9):1905–1914. - PubMed
-
- Baranova A, Gowder SJ, Schlauch K, et al. Gene expression of leptin, resistin, and adiponectin in the white adipose tissue of obese patients with non-alcoholic fatty liver disease and insulin resistance. Obes Surg. 2006;16(9):1118–1125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

