On-line separations combined with MS for analysis of glycosaminoglycans
- PMID: 18956477
- PMCID: PMC4119066
- DOI: 10.1002/mas.20200
On-line separations combined with MS for analysis of glycosaminoglycans
Abstract
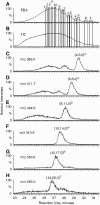
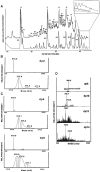
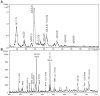
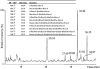
The glycosaminoglycan (GAG) family of polysaccharides includes the unsulfated hyaluronan and the sulfated heparin, heparan sulfate, keratan sulfate, and chondroitin/dermatan sulfate. GAGs are biosynthesized by a series of enzymes, the activities of which are controlled by complex factors. Animal cells alter their responses to different growth conditions by changing the structures of GAGs expressed on their cell surfaces and in extracellular matrices. Because this variation is a means whereby the functions of the limited number of protein gene products in animal genomes is elaborated, the phenotypic and functional assessment of GAG structures expressed spatially and temporally is an important goal in glycomics. On-line mass spectrometric separations are essential for successful determination of expression patterns for the GAG compound classes due to their inherent complexity and heterogeneity. Options include size exclusion, anion exchange, reversed phase, reversed phase ion pairing, hydrophilic interaction, and graphitized carbon chromatographic modes and capillary electrophoresis. This review summarizes the application of these approaches to on-line MS analysis of the GAG classes.
(c) 2009 Wiley Periodicals, Inc.
Figures




References
-
- Adamson JT, Hakansson K. Electron detachment dissociation of neutral and sialylated oligosaccharides. J Am Soc Mass Spectrom. 2007;18:2162–2172. - PubMed
-
- Akiyama H, Shidawara S, Mada A, Toyoda H, Toida T, Imanari T. Chemiluminescence high-performance liquid-chromatography for the determination of hyaluronic-acid, chondroitin sulfate and dermatan sulfate. J Chromatogr-Biomed Appl. 1992;579:203–207. - PubMed
-
- al-Hakim A, Linhardt RJ. Capillary electrophoresis for the analysis of chondroitin sulfate- and dermatan sulfate-derived disaccharides. Anal Biochem. 1991;195:68–73. - PubMed
-
- Alpert AJ. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr. 1990;499:177. - PubMed
-
- Anumula KR. High-sensitivity and high-resolution methods for glycoprotein analysis. Anal Biochem. 2000;283:17–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

