Total synthesis of (+)-superstolide A
- PMID: 18956845
- PMCID: PMC2736345
- DOI: 10.1021/jo801794s
Total synthesis of (+)-superstolide A
Abstract
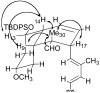
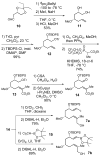
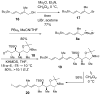
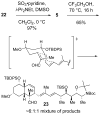
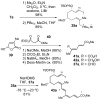
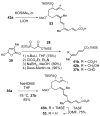
A convergent and highly stereocontrolled total synthesis of the cytotoxic macrolide (+)-superstolide A is described. Key features of this synthesis include the use of bimetallic linchpin 36b for uniting the C(1)-C(15) (43) and the C(20)-C(27) (38) fragments of the natural product, a late-stage Suzuki macrocyclization of 49, and a highly diastereoselective transannular Diels-Alder reaction of macrocyclic octaene 4. In contrast, the intramolecular Diels-Alder reaction of pentaenal 5 provided the desired cycloadduct with lower stereoselectivity (6:1:1).
Figures




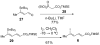









References
-
- D’Auria MV, Debitus C, Paloma LG, Minale L, Zampella A. J Am Chem Soc. 1994;116:6658.
-
- D’Auria MV, Paloma LG, Minale L, Zampella A, Debitus C. J Nat Prod. 1994;57:1595. - PubMed
-
- Zampella A, D’Auria MV. Tetrahedron: Asymmetry. 2001;12:1543.
-
- Yu W, Zhang Y, Jin Z. Org Lett. 2001;3:1447. - PubMed
-
- Solsona JG, Romea P, Urpi F. Org Lett. 2003;5:4681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

