Concerted but noncooperative activation of nucleotide and actuator domains of the Ca-ATPase upon calcium binding
- PMID: 18956892
- PMCID: PMC3236681
- DOI: 10.1021/bi8014289
Concerted but noncooperative activation of nucleotide and actuator domains of the Ca-ATPase upon calcium binding
Abstract
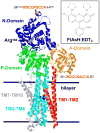
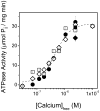
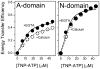
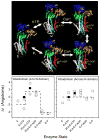
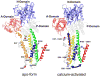
Calcium-dependent domain movements of the actuator (A) and nucleotide (N) domains of the SERCA2a isoform of the Ca-ATPase were assessed using constructs containing engineered tetracysteine binding motifs, which were expressed in insect High-Five cells and subsequently labeled with the biarsenical fluorophore 4',5'-bis(1,3,2-dithioarsolan-2-yl)fluorescein (FlAsH-EDT(2)). Maximum catalytic function is retained in microsomes isolated from High-Five cells and labeled with FlAsH-EDT(2). Distance measurements using the nucleotide analog 2',3'-O-(2,4,6-trinitrophenyl) adenosine 5'-triphosphate (TNP-ATP), which acts as a fluorescence resonance energy transfer (FRET) acceptor from FlAsH, identify a 2.4 A increase in the spatial separation between the N- and A-domains induced by high-affinity calcium binding; this structural change is comparable to that observed in crystal structures. No significant distance changes occur across the N-domain between FlAsH and TNP-ATP, indicating that calcium activation induces rigid body domain movements rather than intradomain conformational changes. Calcium-dependent decreases in the fluorescence of FlAsH bound, respectively, to either the N- or A-domains indicate coordinated and noncooperative domain movements, where both A- and N-domains display virtually identical calcium dependencies (i.e., K(d) = 4.8 +/- 0.4 microM). We suggest that occupancy of a single high-affinity calcium binding site induces the rearrangement of the A- and N-domains of the Ca-ATPase to form an intermediate state, which facilitates phosphoenzyme formation from ATP upon occupancy of the second high-affinity calcium site.
Figures
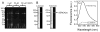


References
-
- Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5:282–295. - PubMed
-
- Moller JV, Olesen C, Jensen AM, Nissen P. The structural basis for coupling of Ca2+ transport to ATP hydrolysis by the sarcoplasmic reticulum Ca2+-ATPase. J Bioenerg Biomembr. 2005;37:359–364. - PubMed
-
- Olesen C, Picard M, Winther AM, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–1042. - PubMed
-
- Toyoshima C. Structural aspects of ion pumping by Ca(2+)-ATPase of sarcoplasmic reticulum. Arch Biochem Biophys. 2008;476:3–11. - PubMed
-
- Toyoshima C, Inesi G. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 2004;73:269–292. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

